There are 1 Hydrogen, 1 Nitrogen and 3 Oxygen atoms in this chemical formula.
What is HNO3?
A bond where electrons are shared between two nonmetals.
What is a covalent bond?
The symmetry of the molecule 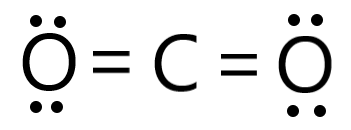
What is symmetrical?
Forces of attraction between molecules.
What is intermolecular or electrostatic forces?
This is not an example of:
What is a chemical reaction?
The number that tells you how many of each element, chemical group or ion are present in a molecule.
What is a subscript?
A bond where electrons are transferred from a metal to a non metal.
What is an ionic bond?
A molecule where electrons are shared equally between the atoms.
What is nonpolar?
The strength of forces between nonpolar molecules?
What are weak intermolecular forces?
(London Dispersion forces)
An example of calculating this important value: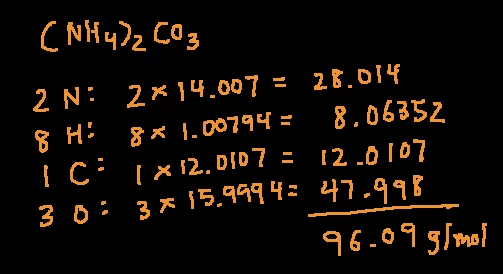
What is molar mass?
The number in front of a chemical formula that tells you the number of molecules or moles of that molecule present.
What is the coefficient?
The lines connecting the elements represent this.
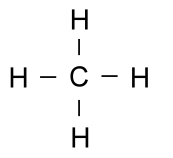
What is two shared electrons?
The polarity of the bonds in the molecule:

What is nonpolar?
The force of attraction that happens between polar molecules, with H, N,O and F.

What are hydrogen bonds?
In order to cancel out the unit moles:
Why was the 1 mol H2O value was placed at the bottom?
The Lewis diagram based on the chemical formula provided is: 
What is the Lewis diagram for CH3Cl?
This movement of electrons in the elements is shown because of a plus and minus in the Lewis Diagram.
What is transferred electrons?
The polarity of the molecule NH3

What is polar?
Molecules with strong intermolecular forces have this melting point. (High, Medium, Low)
What is a high melting point?
This is the type of variable you change when planning to do an experiment.
What is the independent variable?
The total number of each type of atom in the formula 2Ca(NO3)2.
What is 2 Calcium, 4 Nitrogen, 12 Oxygen atoms?
This type of compound can conduct electricity when in solution and has a very high melting point.
What is an ionic bond?
The polarity of this molecule.
What is polar?
Only a small amount of heat energy is needed to break this type of intermolecular force.
What are weak intermolecular forces? (London Dispersion Forces)
This Lewis dot structure is incorrect because:

What is nitrogen has too many electrons?