What charge does a cation have? What charge does an anion have?
Cation = +
Anion = -
Write the name of K2O
What is the prefix for 4?
Write the formula for Chloric acid
HClO3
What is the name for HNO2
Nitrous acid
What are valence electrons?
Electrons on the outermost shell or ring.
Write the name of Ba3P2
Barium Phosphide
Write the name for N2O4
dinitrogen tetroxide
Write the formula for Phosphorous acid
H3PO3
What is the name for HClO3
chloric acid
What does monatomic ion mean? What does polyatomic ion mean?
monatomic ion = an ion with one atom
polyatomic ion = an ion with more than one atom
Write the formula for Cesium nitride
Cs3N
Write the name for NO
Write the formula for Sulfuric acid
H2SO4
What is the name for HCl
Hydrochloric acid
What is this called?
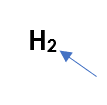
subscript
Write the formula for Cobalt carbonate
Co2(CO3)3
Write the formula for Carbon hexafluoride
SF6
HI
What is the name for H2CO3
Carbonic acid
What is the difference between an ionic bond and a molecular (covalent) bond?
ionic bond = metal + non-metal
molecular (covalent) = non-metal + non-metal
Write the formula for Copper (I) oxide
Cu2O
Write the formula for Trinitrogen disulphide
N3S2
H3BO3
What is the name for H2SO3
Sulfurous acid