What are the reactants in the below reaction?
H2O + CO2 ---> C6H12O6 + O2
H2O + CO2
Carbohydrates are made up of these three elements.
What are CHO?
The lipid shown below is:

A triglyceride
This is the monomer of proteins.
What is an amino acid?
Nucleic acids are made up of these 5 elements.
What is CHONP?
The negative end of the water molecule is located closest to the _________________.
Oxygen atom
These are examples of carbohydrates.
What are Sugars and Starches?
Lipids have longer lasting energy than Carbohydrates. (True or False)
True
DAILY DOUBLE (2 QUESTIONS IN 1)
These bonds hold amino acids together to form this level of protein structure.
Nucleic acids are named after these pentose sugars.
What are ribose and deoxyribose?
How many electrons should Element #17 (Chlorine) have in order to become a negative ion?
18
Starch is classified as this type of carbohydrate.
Polysaccharide / complex carbohydrate
Lipids do not have a repeating monomer structure but can often be distinguished by this straight chain feature.
What is a fatty acid chain/tail?
DAILY DOUBLE (2 QUESTIONS IN 1)
A special class of proteins serve as catalysts for chemical reactions. What are they and how do they accomplish this?
Enzymes...decreasing activation energy
What are the 3 parts of a nucleotide?
1 of 5 nitrogenous bases, a phosphate group, and a pentose sugar (either ribose or deoxyribose)
What will cause an enzyme to lose its bond structure and fall apart. What is this called?
The wrong pH or temperature...This is called Denaturation
Chemical energy: the energy stored in the bonds that hold atoms (matter) together
This hydrophobic coating on plants blocks the passage of water to protect against both water loss and excess water absorption
What is waxy cuticle?
The monomer structure shown below is:
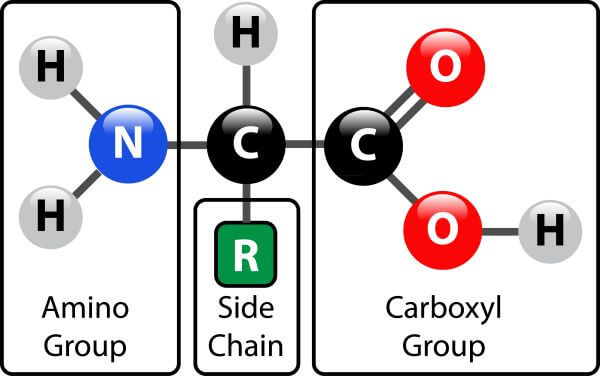
The general branched chain structure shared by all Amino Acids
What is the function of nucleic acids?
Storage of genetic information and...(for bonus points)
DAILY DOUBLE (2 QUESTIONS IN 1)
Which type of reaction is modeled below? Which major biochemical reaction could this represent?
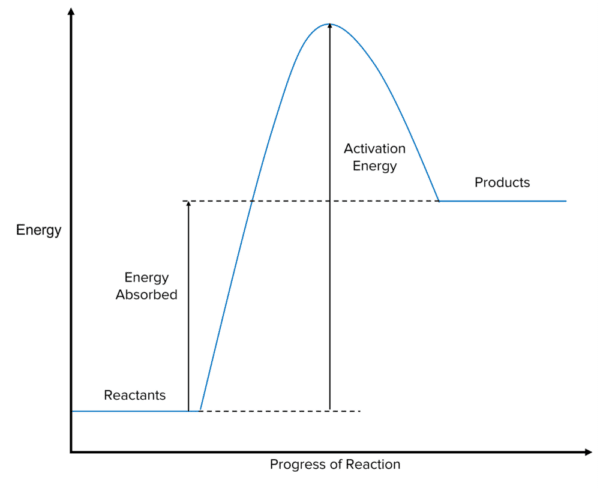
The reaction is endothermic. It can represent photosynthesis.
In what three ways do isomers such as glucose and fructose differ?
Arrangement of atoms, arrangement of bonds, and chemical properties
This class of lipids is characterized as having 4 linked or fused carbon rings, and several of them have a short fatty acid chain/tail
Steroids
This process removes water to combine 2 molecules together.
What is Dehydration Synthesis?
The monomer shown forms which polymer: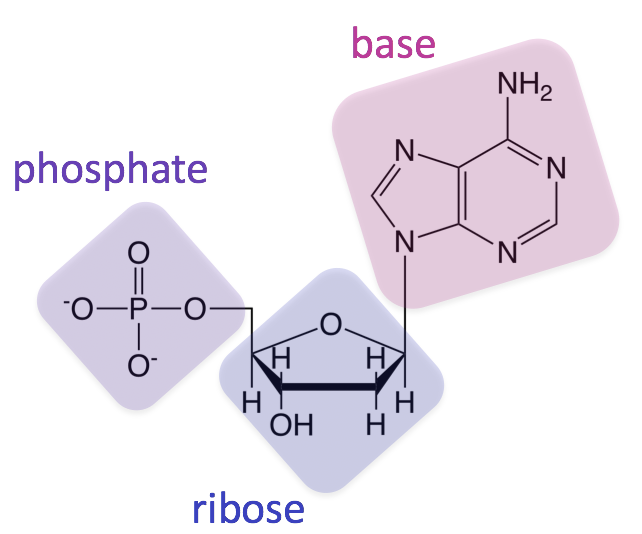
What is a ribonucleic acid?