What is a compound?
a substance made from more than one element joined by a chemical bond
What is Density?
Mass/Volume
Mass of a substance per unit of volume.
What is the definition for property?
particular characteristics of a material such as the materials density, melting point, or color. A change in volume, size or shape of the material does not change its properties.
What are the three states?
Which one has the highest kinetic energy?
Which has the lowest kinetic energy?
Solid, liquid, gas
Highest = gas
Lowest = solid
What is the definition of a particle?
Small structures that make up all matter.
In the molecule C16H18O5 how many elements are there?
3 elements: Carbon, Hydrogen, Oxygen
What is the density of water?
1.0 g/cm3
List two types of physical properties.
Color
Malleability
Odor
State
Density
If you increase thermal energy what does this do to the motion of the particles?
Particles heat up and begin moving faster and break some of the bonds.
What is the definition of malleability?
The flexibility of a material.
Which one is a molecule?
Na
N
CO
Ca
CO
Carbon and Oxygen
If something has a density of 1.2 will it sink or float in water?
Sink
What is a chemical property?
how a material reacts with another substance
(reactivity)
What is happening to the gas particles in a container at room temperature?
The particles are spread far apart and barely touch each other.
What is the difference between a monomer and a polymer?
Monomer is a singular molecular subunit and a polymer is a long chain of smaller units called monomers.
What is the definition of an extended structure?
a varied number of one or more kinds of atoms bonded together to form a larger network.
What is the volume of a cube with a side length of
5 cm?
125 cm3
Which of the following is NOT a property that can be used to identify a material?
Flexibility
Shape
Color
Density
Shape
How is the shape of a substance determined by its state?
Solid particles are stuck together so they hold their shape. Liquid and gas particles move around so they do not hold their shape/can easily change.
What is the definition of temperature?
The measurement of the average kinetic energy of the particles.
In the compound 3CO2, how many of each type of atom?
3C (carbon) and 6 O (oxygen) for a total of 9 atoms
Johnny measures a piece of plastic and determines it has a mass of 20 grams and a volume of 5 cm3 . What is the density of the piece of metal? Will it sink or float?
4 g/cm3
Sink
Which of these substances would be a solid at room temperature?
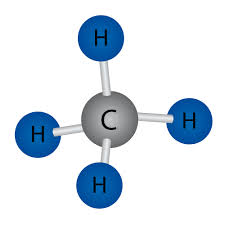
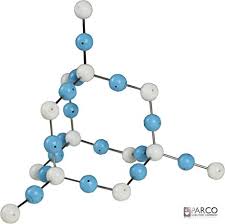

In general, large molecular or extended structures tend to be solids at room temperature.
What happens to the particles when thermal energy is added to a substance?
Explain: Solid>Liquid>Gas
The particles will move more and spread out as the temperature increases because the pressure breaks the bonds.
What are some of the trade-offs associated with purchasing products made from plastic?
Name 3
recyclable
bad for environment
inexpensive
toxic