Classify each of the following

i. element
ii. compound
iii. heterogenous mixture
iv. homogenous mixture
mass of solute / volume of solvent
Describe the most likely way in which two water molecules will interact.
a hydrogen bond will form between the hydrogen of one molecule and the oxygen of another molecule
Often called "Nature’s Kidneys," wetlands act as natural filters by trapping sediment and absorbing excess nutrients before water reaches the ocean. Provide at least one example of a wetland
marshes, swamps, bogs, and/or fens
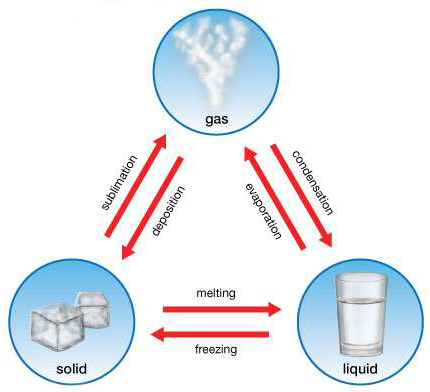
The diagram below shows part of the water cycle. Letters A through D represent processes that take place during the cycle.
Which letter represents transpiration?
B
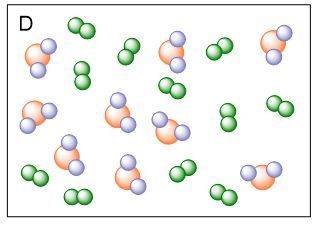
Which image is an example of one element and one compound?



D.
Determine the mass concentration of a salt solution in which 13 g of salt is added to 52 mL of water.
0.25 g/mL
It allows ice to float, insulating the water below.
What three main steps occur in the Sidwell system after water reaches the basement holding tank?
Then label each step with a "P" for physical change or "C" for chemical change.
2 additional filters (P), UV filter (C), addition of blue dye (P)
What is the process where liquid water turns into a gas (water vapor)?
evaporation
This classification of matter is not chemically bonded and has a unified appearance.
Homogenous mixture
2g sucrose is added to 200mL water to form an aqueous solution.
Calculate the mass/volume percent of the sucrose solution. There is a negligible change in volume on addition of sucrose to water.
1%
(mass/volume)x100%
San Diego is less than 1 mile from the Southern Californian coast with temperatures swinging 3 degrees from day to night. Palm Springs is 181 miles from the Southern California coast and has temperature swings of 17 degrees from day to night.
What water properties best explain why coastal cities like San Diego often have a smaller air temperature range than inland cities?
the ocean has a high heat capacity, meaning it heats up and cools down much slower. Therefore, air that is closer to the ocean results in a similar reaction
There are multiple filters in the wetlands area. List one that causes physical change and one that causes chemical change to the water.
Physical - fine sand filter
Chemical - trickle filter
List all the steps to move surface water to ground water.
1. infiltration
2. percolation
What classification of matter is ketchup in a container before shaking it?
heterogenous mixture
10 g HCl is added to 100g water to form an aqueous solution. Calculate the mass percentage of the HCl solution.
9.09%
HCl/solution total x 100
Explain water's ability to dissolve certain substances such as glucose, but remain separate from other substances such as oils.
Water is polar. Glucose is also polar with many hydrogen and oxygen ends available for bonding. Opposites attract with ionic bonds and polarity. Oil is non polar and rather hydrophobic.
Waste water treatment includes primary, secondary, and tertiary. What occurs at each step?
Primary - physically removing large solids
Secondary - biologically breaking down organic materials
Tertiary - purification, removing any nutrients or pollutants
Draw a diagram of part of the water cycle that includes a glacier, a cloud, sublimation, and deposition.
Select all the pure substances:
I. Oxygen gas
II. Mineral water
III. Aluminum sheet
IV. Diesel
V. Soda
VI. Baking soda
I. Oxygen gas
III. Aluminum sheet
VI. Baking soda
Explain what changes and what stays the same when 1.00 L of a solution of NaCl is diluted to 1.80 L.
The number of mass always stays the same in a dilution.
The concentration and the volumes change in a dilution.
How does the bent geometry of water contribute to its polarity?
It prevents dipole moments from canceling out.
In the Blue Plains wastewater lesson, treated and now clean water is released back into the Potomac River. What term describes clean or sewage based liquid being released into rivers or oceans?
effluent
sun and gravity