Only one type of atom
What is an element?
Group 1
What group are the alkali metals also know as?
energy is lost to the surroundings?
What happens to energy in an exothermic reaction?
Delocalised electrons that carry charge through the stricture
Why are metals good conductors of energy?
Ribosome
What part of a cell makes proteins?
2,8,8
How many electrons do the first shells hold if full?
Has 2 electrons in its outer shell
How many electrons in the outer shell of group 2 elements?
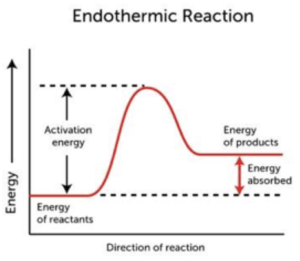
What does the energy profile diagram look like for an endothermic reaction?
ionic bond
What type of bond forms between metals ions and non-metal ions?
Chromosomes or Genetic information
What does the nucleus contain?
Nucleus
Which part of the atom contains protins and neutrons?
Mendeleev
Who created the modern periodic table?
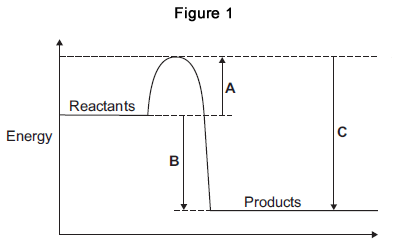 A is correct
A is correct
What letter is the activation energy?
The metal ion and non-metal ion form have a strong electrostatic force of attraction (strong bond) that requires a lot of energy to break
Why do ionic compounds have high melting points?
large surface area to absorb water at a faster rate
What is an adaptation of a root hair cell to aid its function?
Number of protons equal number of electrons
Why does an atom have no overall charge?
He left gaps
What did Mendeleev do for undiscovered elements?
Sports injury pack
What is an everyday use of an endothermic reaction?
The weak intermolecular forces require less energy to overcome
Why do small covalent molecules have low melting points?
Missing a nucleus so it can carry more oxygen
What adaptation does a red blood cell have to aid its function?
Atoms with the same number of protons but a different number of neutrons?
What is an isotope?
Decreasing in reactivity as you go down the group
Describe the reactivity of group 7 (halogens)
Thermal decomposition
Each carbon bonds to 4 other carbons
Why is diamond hard?
Streamlined to move faster
What is an adaptation of a sperm cell?