What is the charge of an electron?
-1 (Negative one)
Sodium Chloride (table salt) is an example of what type of bonding?
Ionic
Name 2 compounds you could react with sulphuric acid to produce copper sulfate.
Copper carbonate or copper oxide
 A cold pack works after popping and mixing the chemicals together. What type of reaction is used: endothermic or exothermic?
A cold pack works after popping and mixing the chemicals together. What type of reaction is used: endothermic or exothermic?
Endothermic
What pH is copper sulfate?
What colour will universal indicator turn into?
pH = 7
Green
Write the electronic configuration of Chlorine.
2,8,7
 Chlorine gas (Cl2(g)) is an example of what type of bonding
Chlorine gas (Cl2(g)) is an example of what type of bonding
Covalent bonding
Electrolysis of sodium chloride will produce these products at the cathode and anode, respectively.
Cathode - Hydrogen gas
Anode - Chlorine gas
Name one example of a combustion reaction found in nature.
Aerobic respiration
Explain why ammonia is a weak base while sodium hydroxide is a strong base
Sodium hydroxide fully ionises in solution, ammonia does not.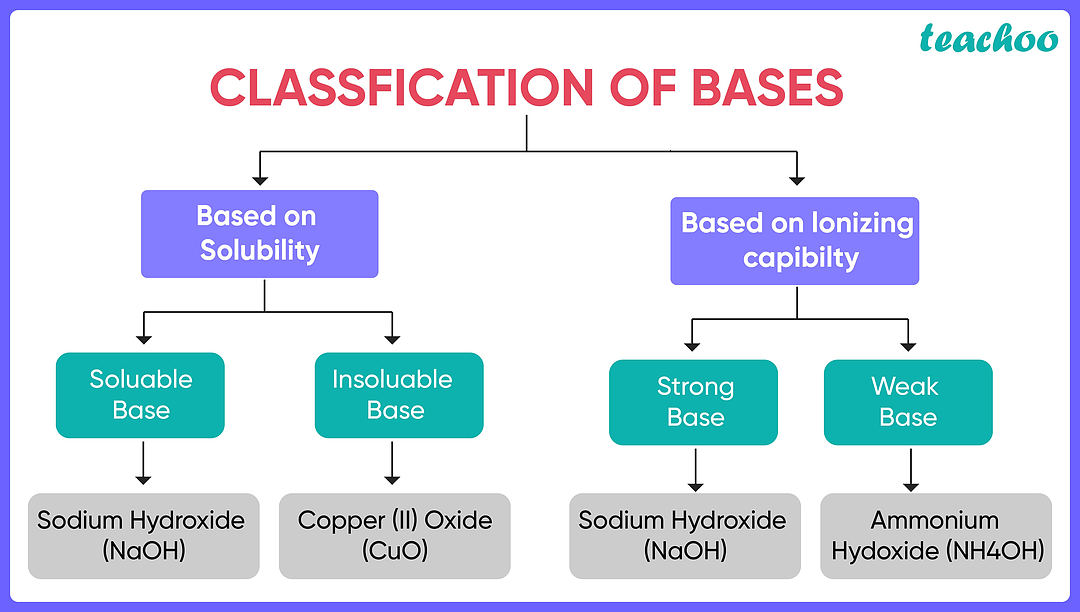
Atoms of the same element, _____ protons and electrons, _______ number of neutrons.
Atoms of the same element, same protons and electrons, different number of neutrons.
Zinc and Sodium metal are examples of what type of bonding?
Metallic Bonding
Complete a half equation for the formation of chlorine gas
2Cl- _> Cl2 + 2e-
What is the catalyst on this graph changing?
Activation Energy (EA)
List all the gas tests and the positive result you're looking for.
Burning splint - Popping sound
Glowing splint - Relighting a splint
Damp litmus paper - Bleached paper
Limewater - Turns cloudy/milky
Draw the model of an atom that Ernst Rutherford disproved.
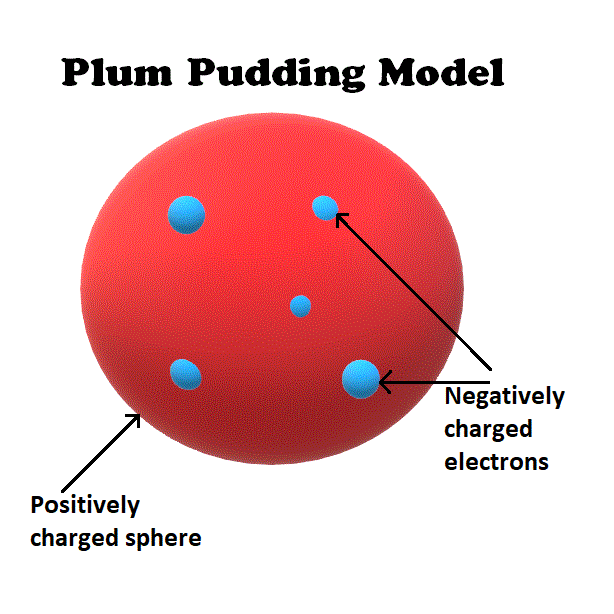
Name an element that shouldn't bond with any chemical.
Any noble gas.
Helium, neon, krypton, radon, xenon.
What is the ionic equation for a neutralisation reaction?
H+ + OH- -> H2O
This graph is showing what type of reaction? Exothermic or endothermic?
Exothermic, the products have less energy (lower on the graph) than the reactants.
 What reactants do I need to produce calcium chloride?
What reactants do I need to produce calcium chloride?
Calcium oxide + hydrochloric acid -> calcium chloride + water
Draw Niel Bohrs model of the atom
Balance this equation:
CH4 + O2 -> H2O + CO2
Methane + Oxygen -> Water + Carbon Dioxide
CH4 + 2O2 -> 2H2O + CO2
Methane + Oxygen -> Water + Carbon Dioxide
Define oxidation and reduction in terms of oxygen.
HIGHER - In terms of electrons.
Oxidation is the addition of oxygen in substances, reduction is the loss of oxygen in substances.
HIGHER - Oxidation is the loss of electrons. Reduction is the gaining of electrons. (OILRIG)
After making a chemical reaction, you see a change in energy of +1000 KJ.
Was this reaction endothermic or exothermic? How do you know?
Endothermic (gained energy)
What steps do you take during the ''Temperature changes'' practical to ensure a valid result?
Use a polystyrene cup to insulate the reaction.