What is the name of the process from liquid to gas?
Evaporation
What does this symbol represent:
Harmful or Irritating
What are valence electrons?
Electrons in the last orbit
What is a pure substance?
A substance that has a fixed chemical composition throughout is called a pure substance
What types of elements are found in the right top corner of the periodic table?
Gases
Which is the difference between chemical and physical properties?
Physical: is a characteristic of a substance that can be observed or measured without changing the identity of the substance.
Chemistry:include color, density, hardness, and melting and boiling points. A chemical property describes the ability of a substance to undergo a specific chemical change.
What does this symbol represent:
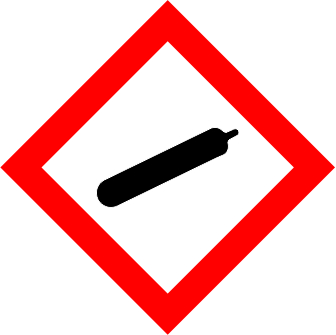
Preassure
What is an isotope?
Same atom with different mass
What classifications does a pure substance have?
Compound and Elements
How are columns and rows named in the periodic table?
Columns: Groups / Family. Rows: Periods
Apart from liquid, solid and gas, what is the 4° state of matter?
Plasma
What is the NFPA safety diamond used to?
Is a system for identifying the specific hazards of a material and the severity of the hazard that would occur during an emergency response
What is the difference between C-12 and C-14?
C-14 has 2 more neutrons than C-12
Explain what is a mixture
Is a material made up of two or more different chemical substances
How many valence electrons are in Family 15?
5
What is the sublimation?
conversion of a substance from the solid to the gaseous state without its becoming liquid.
Which of these symbols represents Toxicity?


Both =)
How many electrons can be found in orbit 4 if all spaces are used?
32
What kind of mixtures are there?
Heterogeneous and Homogeneous
What are some characteristics of metalloids?
What is the volume of a sample that has a mass of 20g and a density of 4g/ml?
v=5ml
Draw the symbol that represents: Dangerous for the environment

What’s the electronic configuration of Ar if its atomic number is 18?
1s2, 2s2, 2p6, 3s2,3p6
Name 3 separation methods:
Separating Funnel, Filtration, Sedimentation, Destilation, Evaporation, Sieving, Magnetic separation, Crustallization, Chromatography.
Name 2 elements of the Boron Family
Boron (B)
Aluminum (Al)
Gallium (Ga)
Indium (In),
Thallium (Tl)