What is an independent variable?
a variable that stands alone and isn't changed by the other variables you are trying to measure. For example, someone's age might be an independent variable.
Liquids have stronger forces of attraction than What?
Gasses
What does homogeneous mean?
the same or similar
examples of homogenous mix.
- Air.
- Sugar water.
- Rainwater.
- Vinegar.
- Dishwashing detergent.
- Steel.
- Cup of Coffee.
- Mouthwash.
what type of mixture does the box contain?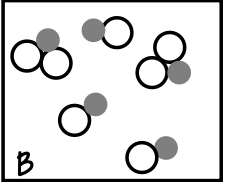
Mixture of Compounds
What is an intensive property?
intensive property is a physical property that does not depend on size or the amount of material. According to the definitions, density, pressure and temperature are intensive properties and volume, internal energy are extensive properties.
What are two classes of matter called?
Pure substance and mixtures
What is a dependent variable?
It is something that depends on other factors.
For example, a test score could be a dependent variable because it could change depending on several factors such as how much you studied.
Which matter is free to move about and fills volume quickly?
Gas
What type of matter is created when to or more elements are chemically combined?
Pure substance
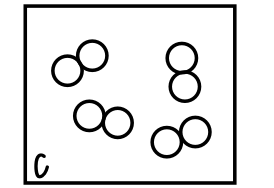
What type of mixture does this box contain?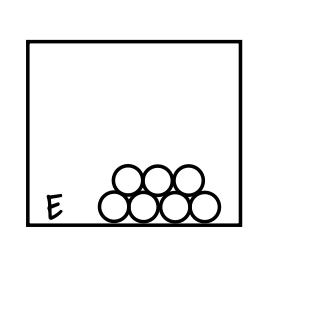
A solid
What is an extensive property?
An extensive property is a property that depends on the amount of matter in something.
Mass and volume are examples of extensive properties.
What are thes examples of? Sodium, chlorine , Sodium Chloride
Pure substances
What are Qualitative Observations?
the observation is based on the observer's subjective interpretation of what they see, hear, smell, taste, or feel.
Examples: My hair is black in color.
Give an example of a physical change
Answers may verry
All changes in state of matter are?
Physical changes
What does this box contain?
Diatomic element
What is a physical Property?
physical property is a characteristic of matter that is not related to change in its chemical composition.
examples
density, color, hardness, melting and boiling points, and electrical conductivity.
Can elements be broken down into simpler particles and remain the same element?
No
When a substance is heated do the particles increase or decrease?
Increase
True or false density is a physical property.
True
What is the formula to calculate mass?
M=VD
What does this box represent?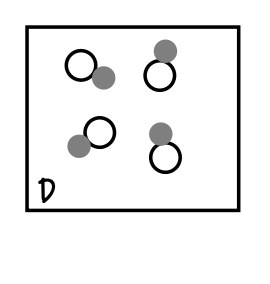
Pure substance compound
What is a chemical property?
change of one type of matter into another type
Examples: flammability, toxicity, acidity, reactivity (many types), and heat of combustion.
Which way should you separate mixture if it's based on particles.
filtration
Which state of matter is less likely to resist compression?
Solids
is oxygen reactivity a chemical property of matter?
Yes
What is the formula for density?
D=M/V
What is an example of a homogeneous mixture?
Air
Is burning wood a chemical change
Yes
How do you separate two liquids in a homogenous mixture.
distillation