Element with atomic number of 7
Nitrogen
Number of Valence electron(s) in a noble gas
8
The substance that donates H+
Acid
All organic compounds contain this element.
carbon
At equilibrium, the rate of the forward and reverse reaction are...
Equal
Element with electronic configuration of 2-8-7
Chlorine
Elements in the periodic table are arranged based on
atomic numbers
The experiment that illustrates the reaction between an acid and a base to make a salt and water
Titration or Neutralization
Name of the nuclear reaction in which one element turns into another element.
transmutation
The increase in concentration of reactants favors which reaction
Forward reaction
Name of experiment and scientist that proved atoms are mostly empty space with a positively charged nucleus
Rutherford's gold foil
The name of the elements in Group 17
Halogens
Color of methyl orange in a basic solution
yellow
Nuclear reaction that splits a large element
Fission
The increase in temperature in an exothermic reaction favors
Backward reaction
Same atomic number different mass number
Isotope
The type of bond that exists in an Aluminum (Al) strip.
Metallic
The Substance that is doing the dissolving
Solvent
Type of hydrocarbon with at least one double bond
What is an alkene
The energy transfer in an electrolytic cell
Electrical to chemical energy
Number of electrons in Al3+
10
The type of bond between Group 1 and oxygen in group 6
ionic
The change in acidity from pH of 5 to pH of 2 represents what increase
1000 fold
Name the functional group in the organic compound shown:
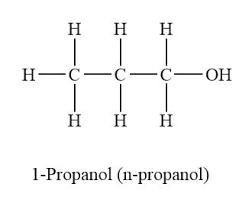
Alcohol
The volume of a 3M acid needed to neutralize 50 mL of a 0.5M base.
8.33 mL (MaVa= MbVb)