What are the three main states of matter?
Solid, Liquid, Gas
Spell: 27 8 19 53 99
CoOKIEs
Which cube is more dense? The left or the right? Explain.

Right. The cubes have the same volume, but the cube on the right has a greater mass (more particles). Therefore, the right cube is more dense (particles are more tightly packed).
What is this piece of equipment called?
Graduated cylinder
Give an example of a substance that can dissolve in water.
Food coloring, salt, sugar,...
Fill in the blanks: In order for something to be considered matter, it must have ____________ and _____________.
Mass, Volume
Spell: 94 9 26 104 53 16 1
Pufferfish
What are the units for density?
grams per milliliter
g/mL
What units are used to measure mass?
grams
What metal produces a light purple color in a flame?
Potassium
What is the center of an atom called?

Nucleus
What is the one letter of the alphabet that does not appear in the Periodic Table?
J
What is the formula for calculating density?
Density = Mass / Volume
Besides milliliters, name 3 units of volume.
Gallons, quarts, pints, fl oz, cm3, etc.
What can you do to increase how fast a substance dissolves in water?
Increase the temperature.
How many protons are in a molecule of water, H2O?
10
(2 Hydrogens, each with an atomic number of 1; 1 Oxygen with an atomic number of 8)
Give an example of an element that is a gas at room temperature.
H, He, N, O, F, Ne, Cl, Ar, Kr, Xe, Rn
What solid object has roughly the same density as the Dawn Dish Soap?
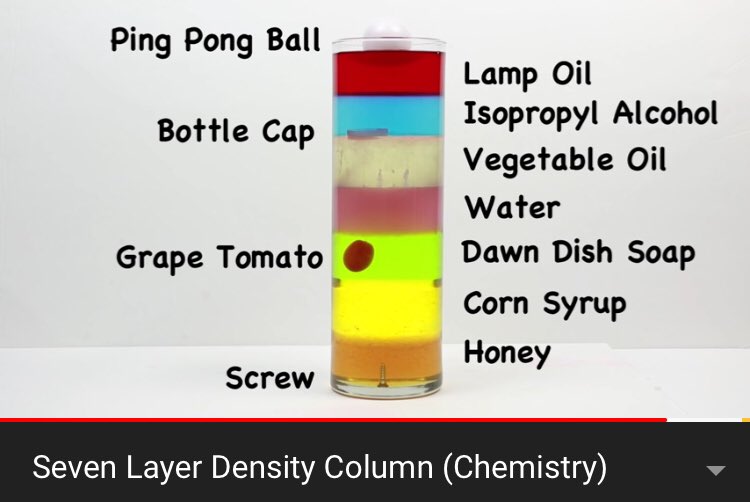
Grape Tomato
How much liquid is in the container, in mL?
:max_bytes(150000):strip_icc()/meniscus01-58b5b2c03df78cdcd8ab8299.png)
24 milliliters
 Where is the hottest part of the bunsen burner flame?
Where is the hottest part of the bunsen burner flame?
Between the inner and outer cones
An atom has 20 protons, 20 neutrons, and 18 electrons. What is its overall charge?
+2
20 protons (positive)
20 neutrons (neutral)
18 electrons (negative)
+20 +0 -18 = +2
Give an example of an element that is liquid at room temperature.
Hg, Br


How much does 1000 mL of liquid water weigh?
1000 g (Density of liquid water is 1 g/mL)
True or False. The density of water does not depend on the temperature. Give an example to support.
False. For example, ice (solid water) is less dense than liquid water.
What type of energy causes electrons of the metal ion to jump from the ground state to an excited state?
Thermal energy from the flame.