The inner part of an atom that contains the proton and the neutron.
What is the nucleus
Consists of only one type of atom
What are elements
Formed when two or more different elements are chemically combined.
What are compounds
The starting materials of a reaction
What are reactants
What element has the symbol Ca?
What is calcium?
These particles have a positive charge and are found inside the nucleus.
What are protons?
Equal to the number of protons; identifies each element
What is the atomic number
Uses element symbols and numbers to show the proportion of each atom in the molecule.
What is a chemical formula
The substances made in a reaction
What are products
How many electrons does an atom of carbon have?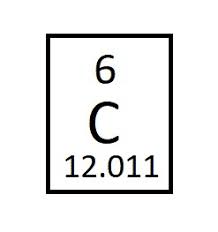
What is 6
These particles are located within the nucleus, and have no charge.
What are neutrons

Which element is shown?
Iron
Hold atoms together and are formed by the valence electrons.
What are chemical bonds
A type of reaction with this format:
A + B --> C
Water is made of these two elements.
What are hydrogen and oxygen?
These particles are negatively charge and are found outside the nucleus.
What are electrons?
What is the significance of 55.845?

What is the average atomic mass
Bond formed when valence electrons are transferred from one atom to another.
Ionic bond
A type of reaction in which energy is required
What is endothermic
What type of bond would form between carbon and oxygen?
What is covalent?
Most of the mass of an atom is located here.
What is the nucleus
Atoms of the same element that have a different number of neutrons.
What are isotopes?
Bond formed when valence electrons are shared.
Covalent bond
A type of reaction in which energy is required.
What is exothermic
The term for a substance that speeds up a reaction but is not consumed in the reaction?
What is a catalyst