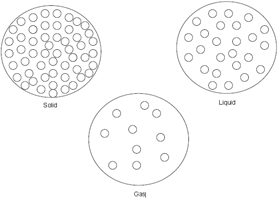
Draw a particle diagram of all 3 states of matter.
What is the ion charge of Phosphorous?
-3
(5 valence electrons so it is closer to 8 and will gain 3 negative electrons)
Name two similarities between the Bohr and Rutherford models
Neutrons and Protons are found in the nucleus.
Electrons are found around the nucleus.
Have neutrons, protons, and electrons.
What type of reaction is the following:
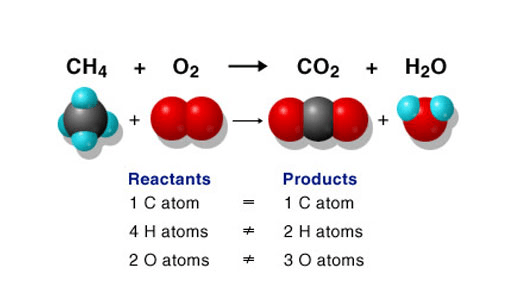
Combustion
There is only one element by itself (1 single) - Oxygen
Is this Fusion or Fission?
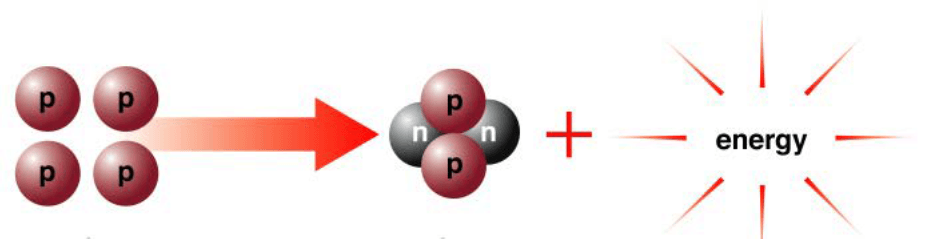
Fusion
The protons are fusing together or combining and releasing energy
List the states of matter from least amount of energy to most.
Solid --> Liquid --> Gas
How many neutrons, protons, and electrons does Argon have?
Neutrons: 40 - 18 = 22
Protons: 18 (equal to the atomic number)
Electrons: 18 (equal to protons)
Describe the main difference between the Bohr model and the Rutherford model.
In the Bohr model, electrons are thought to be found in particular energy orbitals or shells. They are more organized/patterned than Rutherford thought.
In the Rutherford model, electrons are scattered around the nucleus.
What type of reaction is the following:
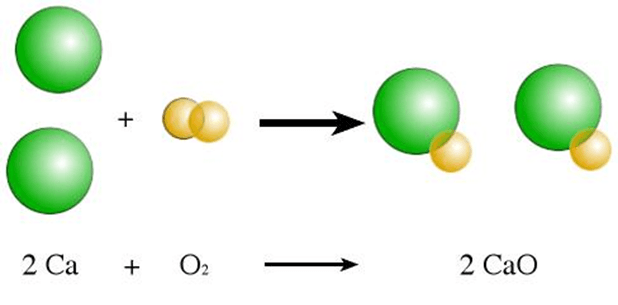
Synthesis
(Two substances are combining to become one type of substance)
Describe Fission
Process of splitting an atom
What is the difference between a solid and a liquid?
Molecules in liquids are spread apart giving them more ability to move. Therefore the molecules are moving faster and have more energy.
What is the ion charge of Magnesium?
+2
(Magnesium only has 2 valence electrons so they will give away both and become positive)
If a substance has a density of 2.4 g/mL is it more or less dense than water?
More dense
(water's density is 1 g/mL)
Balance the following reaction:
___NaBr + ___CaF2 --> ___NaF + ___CaBr2
2NaBr + 1CaF2 --> 2NaF + 1CaBr2
Convert 25F to Celsius
-3.92 C
0.56(25 - 32) = C
Remember order of operations! Parenthesis then multiplication
List the states of matter from least density to most.
Gas --> liquid --> solid
What is the atomic number and atomic mass of Silicon?
Atomic number: 14
Atomic mass: 28
What is the density of an object that has a mass of 2g and a volume of 3.5 mL?
0.57 g/mL
D = mass/Volume
Balance the following:
____ Ag2O --> _____ Ag + _____ O2
2 Ag2O --> 4 Ag + 1 O2
Convert 842K to Celsius
569 C
K - 273 = C
What happens to the molecular structure of a substance when it changes phase?
The structure stays the same, the particles just spread out or move together.
What is the ion charge of Fluorine?
-1
(Fluorine has 7 valence electrons which is closer to 8 so they will gain 1 negative electron)
How does density of a substance change if it goes from solid to liquid?
The substance becomes less dense because the molecules are spreading out.
Rebecca describes a balanced chemical reaction in which 3 atoms of oxygen are needed in the reactants and 4 atoms of oxygen are in the products. Ryan says that according to the Law of Conservation of Matter, Rebecca is incorrect. Who do you agree with?
Ryan
According to the Law of Conservation of Mass, we cannot create or destroy matter so the products and reactants should match.
According to Ohm’s Law when resistance decreases, what happens to current?
It increases
(Remember resistance works like friction - against us!)