What is a pure substance?
ONE type of substance with nothing else mixed in
Physical or chemical property:
Fireworks are able to combust easily

Chemical Property because the fireworks will be changed into a new substance after the reaction

Physical or chemical change:
Cutting your hair

Physical change, it is only a change in appearance and no new substance was made. A change in shape and size.

How can you use the Periodic Table to tell whether something is a metal or nonmetal?
1. If there is a key, you can match the colors to the labels
2. If there is no key, look for the zig-zag staircase that starts at B (boron)
If there is enough heat added, a liquid can _______ into a gas.
Liquid can evaporate into a gas
A container of oxygen gas - pure substance or mixture?
It is a pure substance because there is only one type of compound.
Physical or chemical property:
Lemon juice tastes sour

Physical property because we can use our sense of taste and taste doesn't form a new substance.
Physical or chemical change:
A fallen tree rots and breaks down.

Chemical change because it is breaking down from wood into nutrients in the soil and for other plants.
It cannot be undone and the wood turns into new substances when it breaks down to its nutrients.

What is a metallic bond?
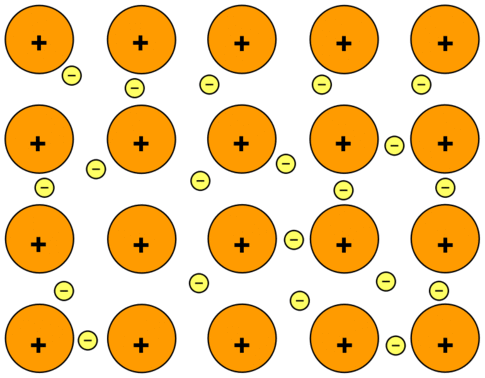
A bond between metals. Positive metal atoms surrounded by negative loose electrons.

Compared to a solid, gas particles are _______

fast
far apart
usually invisible to the eye and less dense
(have more heat energy and a higher temperature)

Pure Substance or Mixture?
Mixture - more than one type of thing. There are different elements and compounds.
Physical or chemical property:
The melting point of stainless steel is 1,510°C

Physical property because it can be measured without forming a new substance, doesn't affect ability to react to other substances.
It is changing state of matter from a solid to a liquid.

Physical or chemical change:
Heat evaporates H2O into steam
Physical change, it is a phase change from a liquid to a gas but it is still H2O and did not form a new substance.
It is a phase change--change in form, but not substance.
Covalent, ionic, or metallic bond:
Li (metal lithium) + F (non-metal fluorine) ions with opposite charges
Ionic bond because it is between a metal and non-metal with opposite charges (positive and negative atoms).
What does the chemical reaction inside of an ice pack cause to happen to the thermal energy inside?
When you crush the pack and start the reaction, the thermal energy decreases because the ice pack gets colder.
Is raisin bran a pure substance, homogenous mixture, or heterogenous mixture?

Heterogenous mixture because it is not mixed and uniform throughout, there are solids and liquid.

Physical or chemical property:
Bleach is a base that reacts to acids like vinegar
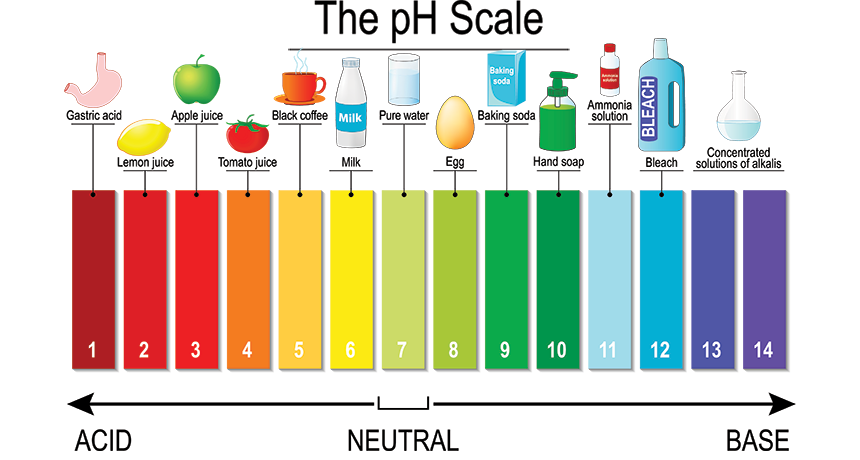
Chemical property because bleach being a base helps it react to acids and can form new substances when it reacts.

Physical or chemical change:
Sodium metal and water are mixed together, producing fire, gas, and a change in color.
Chemical change, combustion (fire), producing gas, and color changes are all signs of a chemical reaction.
It is producing new substances.
HF is a bond between the nonmetal hydrogen and the nonmetal fluorine. Is it a covalent, ionic, or metallic bond?

It is covalent because both are nonmetals and they are sharing electrons.

When two chemicals are mixed, what happened to the contents of the beaker after 10 minutes?
The temperature increased from 25 degrees Celsius to 40 degrees Celsius.
Sugar water - pure substance, homogenous mixture, or heterogeneous mixture?
Homogenous mixture because it dissolves in the water in a uniform way that is the same throughout the glass.
You can't SEE the different parts, it all looks the same.
Physical or Chemical Property:
The high viscosity of oil
Physical Property because it does not affect its ability to react to other substances and can be determined without destroying or changing the substance.
It describes the substance but doesn't have anything to do with forming chemical bonds.

Physical or chemical change:
While cooking chicken, the meat changes color and produces a new, pleasant smell.
Chemical change, there was a change in temperature, change in color, and change in smell.
It cannot be undone and cooked chicken has different properties from raw chicken.

Covalent, ionic, or metallic bond:
Au-Ag alloy between gold and silver
Metallic bond because both are metals (to the left of the staircase).
Compare and contrast the particle movement of water as a solid, liquid, and gas.
The particles in water as a solid move much slower, when it melts into liquid it gains energy and particles move a little faster, and when it evaporates into a gas the water molecules move much faster because it has the most amount of heat energy.