Elements in one of these have similar chemical properties.
What is a group (or family)?
This principle states that if no matter is added or taken away, or in other words, in a "closed system," the mass does not change.
What is Conservation of Mass?
Compare the product of a chemical reaction to the reactants.
What is a substance that was not present before, having different properties?
We use a scale to find this, although the correct way is to use a balance.
What is mass?
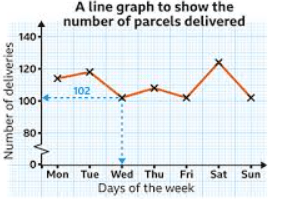 The independent variable in this graph
The independent variable in this graph
What is "days of the week"?
Elements in this group are unreactive gases.
What are the noble gases?
Based on the principle of Conservation of Mass, if the mass of the system increases, we know this.
What is matter must have been added?
Bubbles forming in a reaction mixture than is not being heated.
What is evidence of a chemical reaction?
We measure it with this device
What is volume?
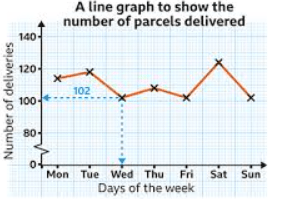 Number of deliveries on Tuesday
Number of deliveries on Tuesday
What is 119 or 120?
Elements in a period have the same number of these.
What are electron shells (or electron energy levels)?
If 30 g of metal is heated in air, and the final mass is 35 g, this amount of oxygen must have combined with the metal.
What is 5 g?
A color change.
What is evidence of a chemical reaction?
The volume of colored water in this graduated cylinder, including one estimated digit

What is anything from 25.0 to 25.3?
In this year, the best fit for Local Index reached 40.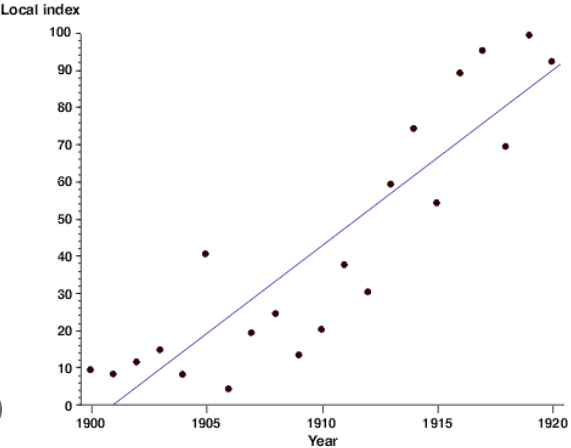
What was 1910?
This group contains the most highly reactive metals.
What are the alkali metals (Group1)?
If the masses of water and Alka-Seltzer combined are 100 g, and when they react bubbles of gas escape leaving 98 g of solution after the reaction, this is the mass of gas that escaped.
What is 2 g?
A precipitate forming from solution.
What is evidence of reaction?
Name of this item of lab equipment.

What is a beaker?
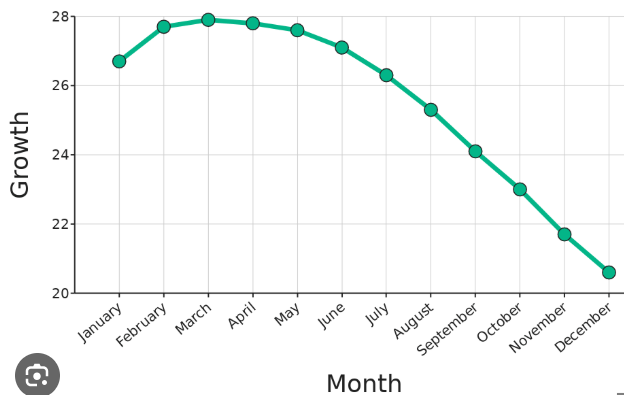 This month showed the greatest growth.
This month showed the greatest growth.
What is March?
This group contains the most highly reactive nonmetals.
What are the halogens (Group 17)?
1 g of water is placed in a 20 g glass tube, which is then sealed shut. The tube is heated until the water all evaporates. This is the mass of the tube after heating.
What is 21 g?
Bubbles forming in a liquid that is being heated at its boiling point.
What is not evidence of a reaction (just boiling)?
This is what we know when the pans of a balance are level
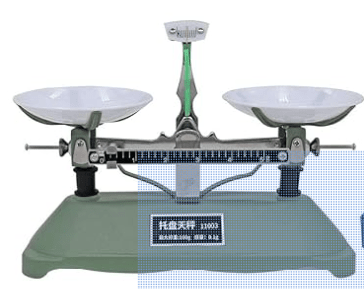
What is the masses on both sides are equal?
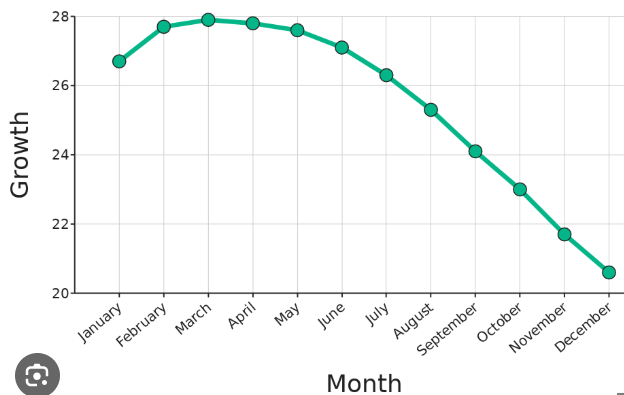 Growth was 24 in this month.
Growth was 24 in this month.
What is September?