Is luster a physical or chemical property?
Physical
Oxygen has ___ valence electrons.
6
A vertical column on the periodic table is called a
Group or family
Carbon dioxide
CO2
Na2O
Sodium Oxide
Is the mass of an iron nail an intensive or extensive property?
extensive
The element with this electron configuration 1s2 2s2 2p6 3s2 3p3 has how many valence electrons?
Phosphorus has 5 valence electrons
The element in group 4, period 5
Zr
Potassium bromide
KBr
Mg3N2
magnesium nitride
A metallic bond is made up of a matrix of positive ions and the negatively charged __________.
Sea of electrons
Draw the Lewis dot structure for water, H2O

Going down the periodic table, atomic radius increases or decreases?
increases (the atoms get bigger)
Copper(II) phosphide
Cu3P2
AlPO4
Aluminum phosphate
Metals have many properties such as malleability and conductivity because the electrons are ______.
Mobile, or able to move around.
In the compound PCl3, P has ___ lone pairs and ___ shared pairs of electrons.
one lone pair and 3 shared pairs
Going across the periodic table, atomic mass increases or decreases?
increases (atoms get heavier)
Sodium nitrate
NaNO3
FeCl3
Iron III Chloride
The name of the property that allows metals to be stretched into wires
Ductile
The shape of a carbon dioxide molecule
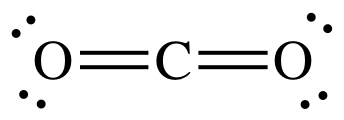 linear
linear
Going across the periodic table, electronegativity increases or decreases?
increases (Fluorine is the electron-stealing bully!)
Copper IV Sulfide
CuS2
C3Cl8
tricarbon octachloride