What is the name of group 18
Draw the orbital diagram for oxygen
___ ___ ___ ___ ___
1s 2s 2p
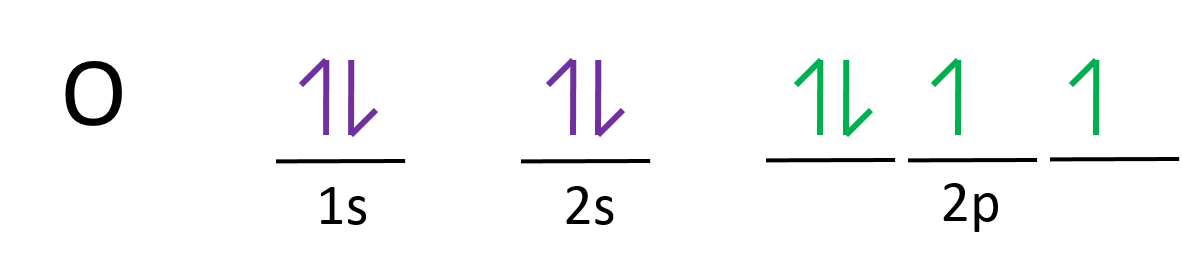
How many valence electrons does an atom of As contain?
As has 5 valence electrons (in group 15 or 5A)
Does the graph below represent a periodic trend?
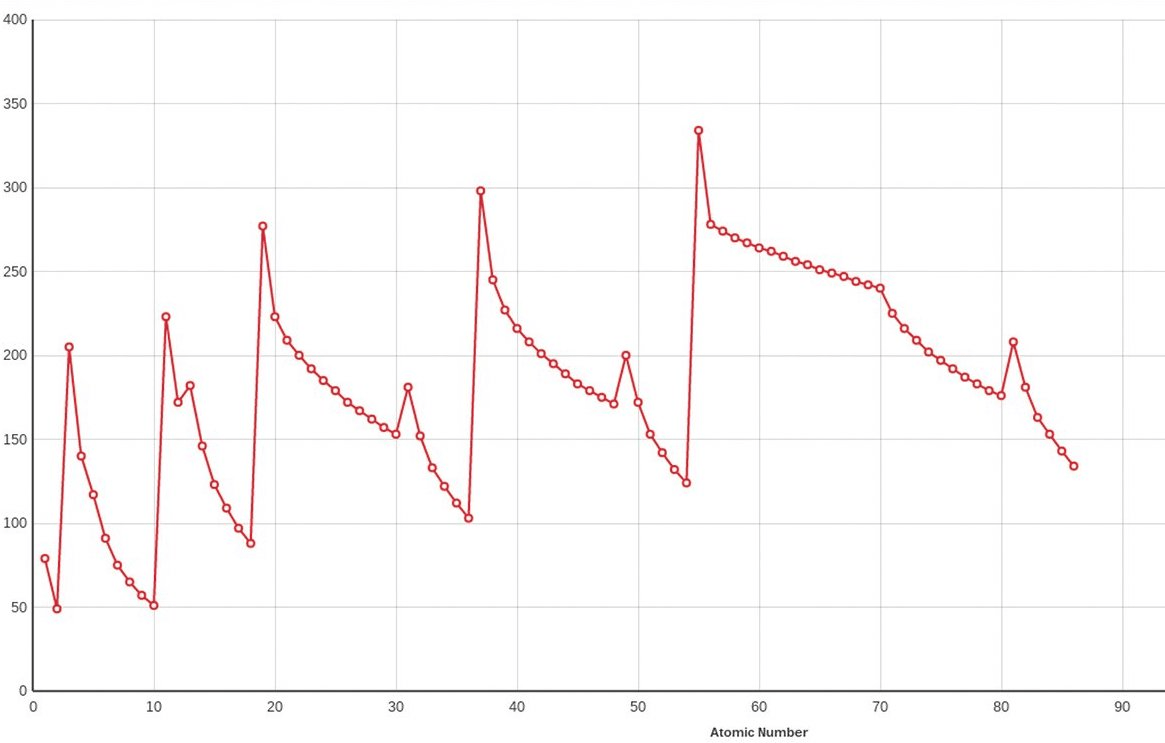
Yes
Draw the orbital diagram for sulfur

What groups are the included in the transition metals?
groups 3-12
Provide the period, group, and block for the following electron configuration:
[Kr]5s24d8
(Element is Pd)
period: 5
group: 10
block: d
How many valence electrons does an atom in the alkaline earth metals family contain?
2
Which has a larger atomic radius: Ti or Y
Y: atomic radius increases down a group and decreases across a period, we also know that helium has the smallest radius so the further an element is to He the larger it will be
Does the graph below represent a periodic trend?
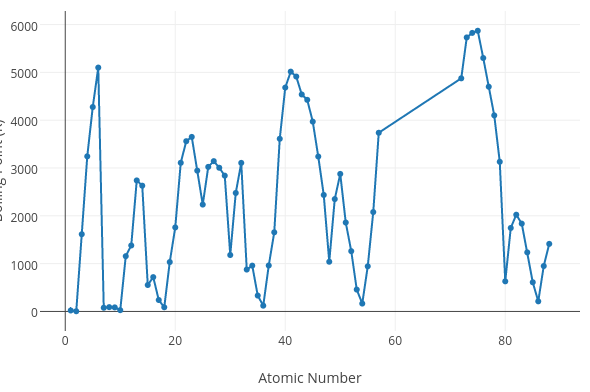
No
List the alkali metals
all of group 1, except H
(Li, Na, K, Rb, Cs, Fr)
Write the electron configuration of Cl
1s22s22p63s23p5
In what energy level are the valence electrons in As located?
the 4th energy level (period 4)
Which element has a lower ionization energy?
S or Si
Si: ionization energy increases across a period, both elements are in period 3 so the element further to the left would have a lower ionization energy (also Si is further from He)
What element has the higher electronegativity?
N or P
N (nitrogen)
Sort the following into metals, nonmetals, and metalloids: Br, Na, B, Mn, Te, O
Metal: Na & Mn
Nonmetal: Br & O
Metalloid: B & Te
Write the noble gas notation for arsenic (As)
[Ar]4s23d104p3
The number of valence electrons contained by the noble gases.
8, except He which has 2
Which is more reactive: Cl or Se
Cl: both are nonmetals so we are comparing to how close the element is to fluorine (F), the most reactive nonmetal. Cl is closer to F than Se making it more reactive
Side note: *Remember a noble gas will always be the least reactive*
As you move across the transition metals in the 4th row what energy level is being filled?
3rd energy level (all end in 3d?)
Which of the following is a representative (main group) element?
Nb, Cu, Li, Hg
Li
Se2- would have an electron configuration that looks like which neutral element?
Kr: the negative 2 charge means that 2 electrons were added to the atom, Se has an atomic number of 34 so when neutral it contains 34 electrons. Once the two extra electrons are added it will look like element 36. (Remember ions tend to look like noble gases because they have stable electron configurations).
An element with 7 valence electrons belongs to what group?
Halogens
Which is more reactive: Ni or Zr
Zr: both are metals so we are comparing how close they are to francium (Fr). Since Zr is closer to francium it will be more reactive than Ni
The names of Ms. Zibart's cats