What device do we use to measure the mass of a substance?
Balance or Scale
How many protons are in Carbon?
6 (Atomic number)
What are the vertical columns in the Periodic Table Called?
Groups / Families
What is the name of this compound NaCl?
sodium chloride
What is Ms. Zapien's first name?
Getsemani
How close a value is to the true/correct value
 What subatomic particles are inside the nucleus?
What subatomic particles are inside the nucleus?
protons and neutrons
Oxygen is a: ________
(metal/nonmetal/metalliod)
Nonmetal
What is the name of the compound N2O4 ?
dinitrogen teraoxide
What type of reaction is called when there is a RELEASE on energy?
exothermic
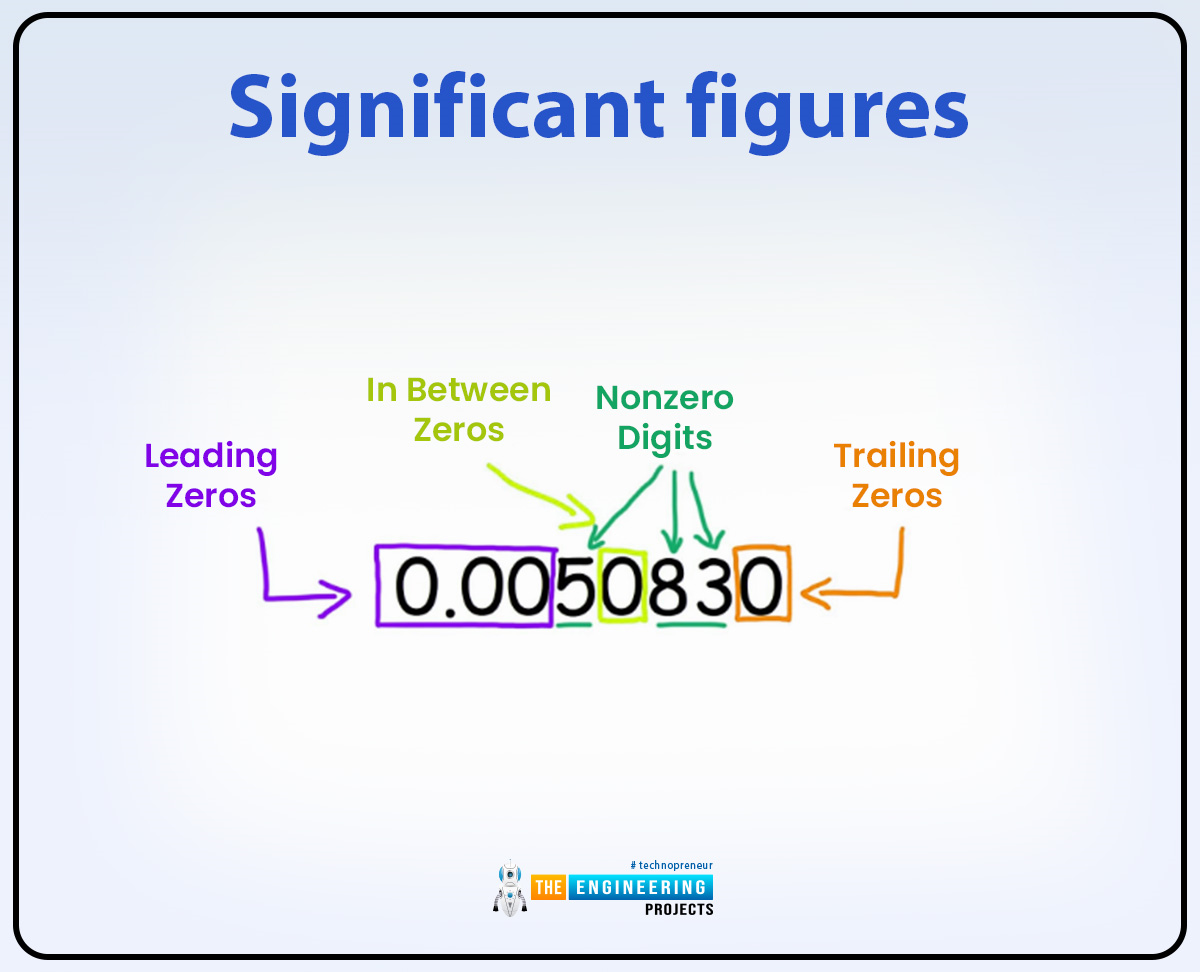 How many significant figures are in 0.00310580 ?
How many significant figures are in 0.00310580 ?
0.00310580
6 Sig Figs
First:2
Second: 8
What element has this eletron configuration?
1s22s22p63s23p2
What is the compound that is formed from Lithium (Li) and Nitrogen (N)?
Li3N
What element is in Period 5 group 5A?
Antimony (Sb)
What is the percent error for a measured value of 23.5 mL when the theoretical value is 25.0 mL?
6%
Why are these isotopes of each other?
C-12 , C-13 , C-14
Same element, different mass
(same protons, different neutrons)
How many electrons fit in the p subshell ?
(think of Electron Configuration)
6 electrons
2p6
IONIC Bonding
Convert 74.3 cm to m.
Given: (100 cm = 1 m)
0.743 m
or
7.43 x 10-1 m
Name two ways that new elements were made
Fusion: atoms merge
Fission: atoms split apart
Supernova: stars explode
Man Made: particle accelerators
Which element is larger in radius?
Potassium (K) or Calcium (Ca)
Potassium (K)
- Radius decreases as you go right
How many dots would be drawn around the Lewis Dot Structure for Oxygen?
6 (group #6)
Is this molecule polar or nonpolar? Explain why.
Polar,
since it is not symmetrical and is not the same on both sides