A very strong intermolecular force that exists only between H, and O, F, or N
The name of the compound LiCl
What is lithium chloride
The product of the synthesis reaction between H2 and Cl2
H2 + Cl2 --> ______
HCl
When a process releases energy (in the form of heat) to its surroundings
What is an exothermic process
A type of acid that will completely dissociate into ions.
Examples
HCl --> H+ + Cl-
What is a strong acid
The weakest intermolecular force --exists between all molecules
What is London Dispersion Forces
The name of the compound N2Cl4
Wht is dinitrogen tetrachloride
Balance the following chemical equation
__Fe + __Cl2 --> __FeCl3
2Fe + 3Cl2 --> 2FeCl3
This is the minimum energy required to be added to the reactants for a reaction to happen
What is activation energy
The products of the neutralization reaction between HBr and LiOH
HBr + LiOH --> ______ + ______
What are HOH and LiBr2
HBr + LiOH --> HOH +LiBr2
This type of intermolecular force is responsible for surface tension in water
What is hydrogen bonds
This type of bond can be found in a molecule of CH4
What is non polar covalent
The molar mass of benzene: C6H6
What is 78.11g/mol
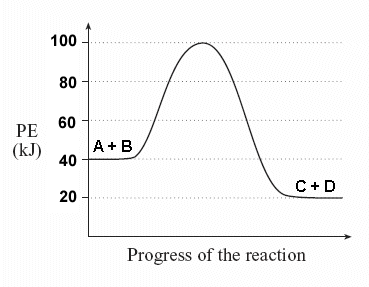
Draw the potential energy diagram:
Energy of reactants = 40kJ
Energy of products = 20kJ
Activation Energy = 60kJ
There are two types of compounds in chemistry: those with a low pH called _____ and those with a high pH called ______
What is an acid (Low pH)
What is a base (High pH)
This type of ion is formed when an atom loses an electron to become positive
What is a cation
The chemical formula for magnesium nitrate
What is Mg(NO3)2
How many particles are in 3.5 mol of NaCl?
What is 2.108x1024 particles
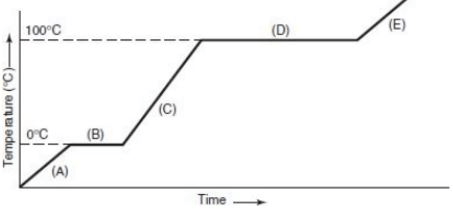
According to the heating curve, these positions are the phase changes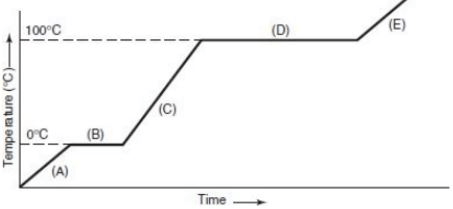
What is positions B and D
The pH of a solution with [H+] of 3.2x10-4
What is 3.49
pH= -log(3.2x10-4)
A type of compound formed between a metal and a non metal
What is an ionic compound
The chemical formula of the compound iron (II) carbonate
What is FeCO3
The mole ratio between chromium and sulfur in the equation:
16Cr + 3S8 --> 8Cr2S3
What is 16mol Cr = 3mol S8
What is 123,310J or 123.3kJ
The pH for neutral
What is 7