Which subatomic particle has a positive charge?
Proton
How many electrons does a neutral atom of Fluorine (F) have?
Which element has an atomic number of six (6)?
Carbon (C)
Name any element with two (2) valence electrons.
*Look at group 2 on the periodic table
A ____ electron is found in an elements outermost orbital shell; these electrons are useful in chemical bonding.
Valence
Which two (2) subatomic particles are contained in the nucleus?
Proton and Neutron
How many valence electrons does a neutral atom of Fluorine (F) have?
Seven (7)
Atomic ____ is equal to protons plus neutrons.
Mass
Every ____ is an atom that has an uneven number of protons and electrons (e.g. Li+, O2-, F-)
Ion
If one side of a scale held one-thousand (1,000) electrons and the other side held one (1) neutron, which side would be heavier?
The one (1) neutron
Which subatomic particle has the smallest mass?
electron
The net charge of any atom is equal to ____ minus ____
Protons, electrons
What element is pictured here?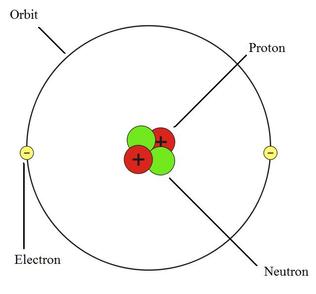
Helium (He)
Name every element on the periodic table that has fewer protons than Lithium (Li)?
Hydrogen (H) and Helium (He)
List the number of each subatomic particle for a neutral atom of Hydrogen (H).
Proton = 1
Neutron = 0
Electron = 1
Which subatomic particle does not have any electrical charge?
Neutron
Draw the Lewis Electron Dot Diagram for Sulfur (S)
What is the two letter symbol for Chlorine?
Cl
Name any element that has more neutrons than protons (Hint: Atomic Mass = Protons + Neutrons)
*See Periodic Table
What is the atomic mass of an element with fifty (50) electrons, twenty-five (25) neutrons, and twenty (20) protons?
forty-five (45)
True or False: Every element has the same number of protons and neutrons.
False
A gold ion that has a charge of 2+ (Au2+) has how many electrons?
Seventy-seven (77)
What element has eleven (11) electrons, twelve (12) neutrons, and eleven (11) protons?
Sodium (Na)
Name any element on the periodic table that has more protons than neutrons (Hint: Atomic Mass = Protons + Neutrons)
Hydrogen (H)
What is the net charge of an element with fifty (50) electrons, twenty-five (25) neutrons, and twenty (20) protons?
negative thirty (-30)
