What are the products?
Classify: Pb(NO3)2 + K2CrO4 ---> PbCrO4 +2KNO3
What is double replacement?
What holds together water molecules?
Hydrogen Bonds
is a flashlight shining light endothermic or exothermic
exothermic
Chemical equations must be balanced to satisfy the ___________.
What is the Law of Conservation of Mass/Matter?
What is the difference between endothermic and exothermic?
Endothermic - absorbs energy
Exothermic - releases energy
Classify: P4 + 5O2 ---> P4O10
What is synthesis?
Surface tension in water is due to:
Hydrogen bonding
What is the heat of reactants and products?
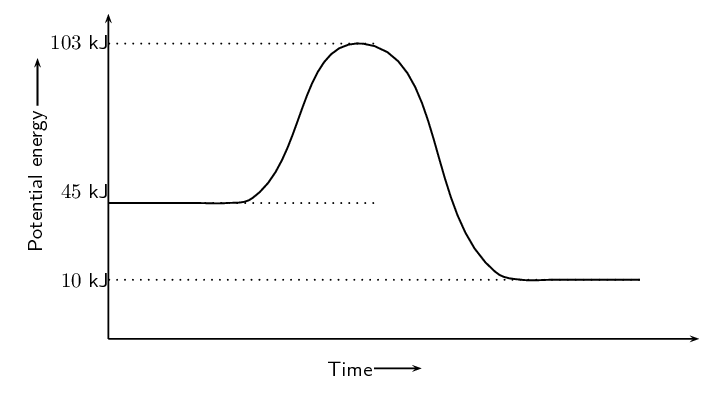
reactants 45 kJ
Products 10 kJ
The number of oxygen atoms
4 Al2(CO3)3
36 oxygen atoms
What is the difference between Q and c in
Q = mc (deltaT)
Q is heat energy lost or gained
c is specific heat
Classify: 2H3PO4 ---> H4P2O7 + H2O
What is decomposition?
Which of the following are insoluble?
NaCl CO2 KBr CCl4 SF6
CO2 CCl4 SF6
Covalent compounds
What is the activation energy?

58 kJ
What conversion factor would you use to convert 3.5 molecules of water to moles? Be specific
1 mol/6.02 x 10^23 molecules
Define solute and solvent
Solute is being dissolved
Solvent is doing the dissolving
In a combustion reaction, what is always produced?
What are CO2 and H2O?
Name THREE ways to help dissolve a solid and
TWO ways to dissolve a gas
Solid: increase temperature, stir, decrease particle size
Gas: decrease temperature and increase pressure
Which are endothermic?
Melting
Freezin
Evaporation
Condensation
Melting and Evaporation
What are the melting and boiling points of this sample?
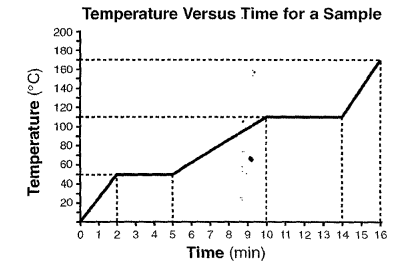
melting 50 degrees Celsius
Boiling 110 degrees Celsius
What is a mole ratio?
Conversion factor using coefficients from a balanced equation
Balance and classify: C4H10 + O2 --> H2O +CO2
2C4H10 + 13O2 --> 10H2O + 8CO2
2, 13, 10, 8
combustion
Determine the mass of a 12 M, 300 mL solution of CO2.
x/(0.300 L) = 12 M
x = 3.6 mol
3.6 mol xx (44.01 g)/(1 mol)
158.44 grams
What is the heat of reaction? Endo or Exo

-35 kJ
Exothermic
Which segment(s) are phase changes?
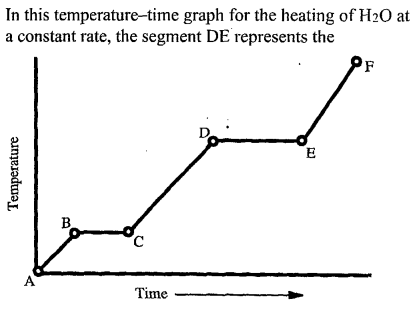
BC and DE