An educated guess
What is the difference between Mass & Weight?
Mass is amount of matter, weight is determined by the strength of the gravitational body the matter is on.
How many digits are significant in 00205.00 and state why.
5, leading 0s are not significant, captive always are, and lagging are only significant when a decimal is present.
The first western philosopher recorded to have proposed the concept of the atom.
Democritus
What is the name, charge, and location of each of the three subatomic particles?
Nucleus contains a neutral neutron and a positive proton.
The electron cloud surrounding the nucleus has negative electrons.
If you miss a day of class, what should you do when you come back?
Request missing assignments and schedule make up quizzes/test with your teacher.
What is a scientific law?
A theory that has been tested over and over again and found to be true without exception.
Is marble homogeneous mixture or a heterogeneous mixture?
Heterogeneous, you can visibly see sections with different chemical compositions.
How many sigfigs are in 3.65*105?
3
The cathode ray tube experiment was used to propose the existence of this subatomic particle.
Electron
What is the mass number of the isotope, Oxygen-17
17
Describe the rules of capitalization for element symbols.
Only the first letter of each symbol in an element should be capitalized.
What is the difference between a theory and a conspiracy theory?
Theories have been tested and are agreed on by consensus in the scientific community.
State definite or indefinite shape and volume for Solids, Liquids, & Gases.
Solids - definite shape and volume
Liquid - indefinite shape and definite volume
Gas - indefinite shape and volume
2 graduated cylinders is a count with no uncertainty. When reading a graduated cylinder, there is an estimated last digit that is uncertain.
JJ Thomson's model of the atom is called the....
plum pudding or chocolate chip cookie model
2 protons, 2 neutrons, and 1 electron
What is the charge of a cation/anion.
Cations are positive, anions are negative.
List the five steps of the scientific method discussed in class.
1. Observe
2. Hypothesis
3. Experiment
4. Analysis
5. Conclusion
Relate the formula 10g H2O + 5g O2 -> 15g H2O to the law of conservation of mass.
Matter cannot be created or destroyed. There were 15g of matter before and after the reaction, the atoms were just rearranged.
Explain the rules for determining # of Sig figs for addition/subtraction & multiplication/division.
Add/Sub are determined by fewest decimal places. Mult/div are determined by fewest sig figs.
What were Dalton's 4 postulates?
1. All elements are made of indivisible atoms
2. All atoms of the same element are identical
3. Atoms of different elements combine in whole number ratios
4. Chemical reactions occur when atoms seperate, join, or rearrange
The modern technical definition for atomic mass units is....
1/12th the mass of a carbon-12 atom
Anions tend to belong to what group?
Non-metals
Teacher's Discretion
1. Observe
2. Hypothesis
3. Experiment
4. Analysis
5. Conclusion
Descirbe the physical properties and changes, as well as the chemical properties and changes that can be demonstrated by a burning candles.
1. Melting Wax shows a physical change in state of matter
2. Physical properties would be the melting POINT of the wax
3. Chemical change is the wick burning to smoke
4. Chemical property would be flammability of the wick
Give the answer for both equations with appropriate sigfigs. 2.2+20=x1 3.00*02=x2
x1=22
x2=6
In detail, describe the set up, results, and conclusions of the gold foil experiment.
Radioactive sample in a lead box shot an alpha particle at gold foil with sensors around the foil. Most passed through but some refracted and some reflected. This led to the orbital theory of a solid positive nucleus with electrons orbiting around it. (The atom is mostly empty space.)
What is the general formula for calculating atomic weight? (not atomic mass)
%1Mass1+%2Mass2+.....
Complete this chart. You may use your periodic table.
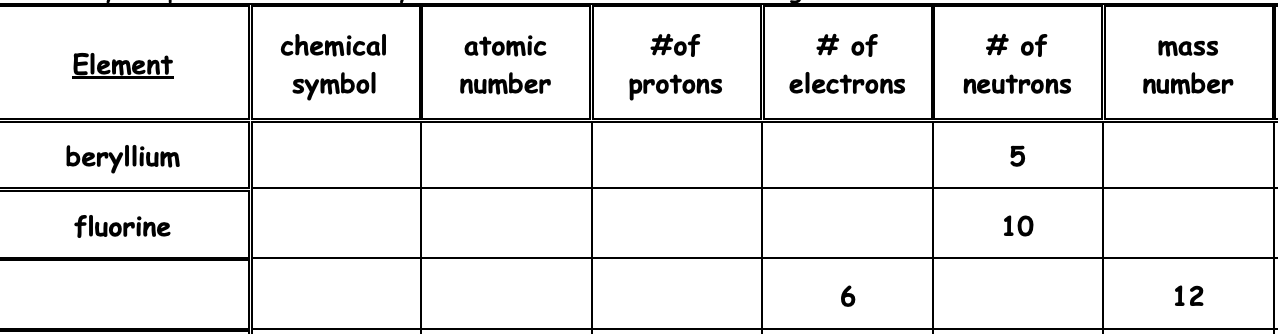
Teacher Approval