What three subatomic particles make up atoms?
Protons, Neutrons, and Electrons
This type of bond forms when electrons are transferred from one atom to another.
ionic bond
______ ______ change substance into different substances by breaking chemical bonds and forming new chemical bonds, rearranging atoms in the process.

Chemical reactions
When potassium chlorate is heated and produces potassium chloride and oxygen gas, the reaction is classified as this.
decomposition
Name the compound MgO
magnesium oxide
List the charge for each of the subatomic particles. Tell which is which.
Protons are positive
Neutrons are neutral
Electrons are negative
NaCl is held together by this type of bond.
ionic
In the following reaction, what is the reactant(s) and what is the product(s).
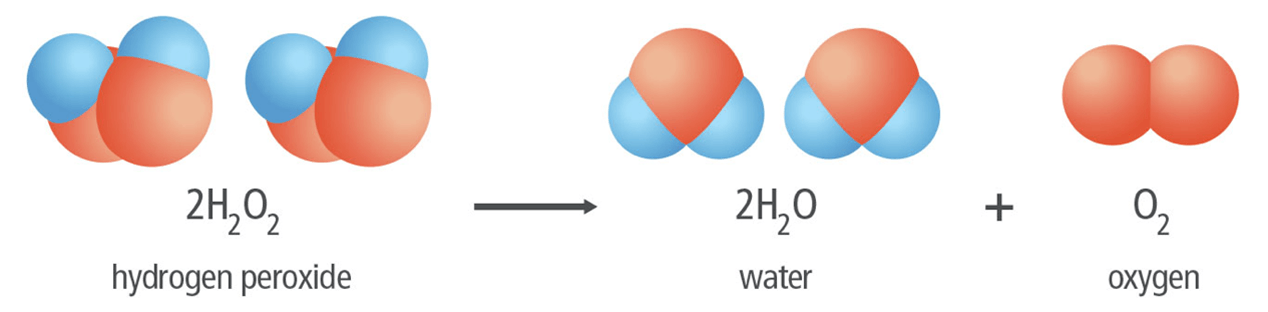
Hydrogen peroxide -> Water and Oxygen
Magnesium and oxygen combine to form magnesium oxide. This is an example of what type of reaction?
synthesis
Balance this combustion reaction of a liquid hydrocarbon:
C₅H₁₂ + O₂ → CO₂ + H₂O
C₅H₁₂ + 8O₂ → 5CO₂ + 6H₂O
Label the following diagram:
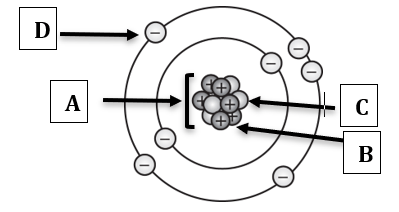
A) Nucleus
B) Proton
C) Neutron
D) Electron
A molecule that ends in “-ide,” like CO₂ or H₂O, almost always contains this kind of bonding.
covalent bonding
According to the ________________ ___ _______________, the product(s) of this reaction should have _______carbon, ________oxygen, ________Sodium, and _________ hydrogens.
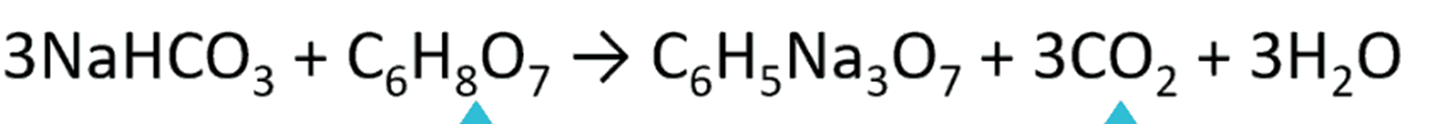
conservation of matter, 9, 16, 3, 11
What kind of reaction is
Cu + 2AgNO₃ → 2Ag + Cu(NO₃)₂
single replacement
When the atoms in the grain dust react and combust with oxygen in the air, and the reaction occurs, describe what happens to the number of atoms in the reaction.
It stays the same according to the law of conservation of mass
The _____ is equal to the number of _____ in the nucleus and determines the ______ of an element.
Atomic number, protons, identity
Write the formula for the compound formed between aluminum and sulfate ions
Al₂(SO₄)₃
List FOUR indicators that a chemical reaction has occurred
heat is released
light emission
gas/bubbles
permanent color change
formation of a precipitate
This reaction type is often confused with decomposition because one of its reactants breaks apart into radicals before reacting. In the reaction:
C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O,
the initial step involves bond cleavage, but the overall reaction is classified as this type, which always releases large amounts of energy and requires oxygen as a reactant.
combustion
Describe the significance of the roman numeral in the following compound name: copper(I) carbonate.
The Roman numeral in a compound name indicates the oxidation state (charge)
Which element has 84 protons in its nucleus
Polonium
What is best represented by this model?

How Lithium and Fluorine bond
Which reaction type always produces CO₂ and H₂O when a hydrocarbon reacts with O₂
Combustion
When aqueous ammonium hydroxide is produced in a double replacement reaction, it immediately breaks down into ammonia gas and water. This makes the overall process appear to be a single reaction but is really these two types occurring back-to-back.
double replacement and decomposition
Balance this double-replacement reaction that forms a precipitate:
Al₂(SO₄)₃ + Ca(OH)₂ → Al(OH)₃ + CaSO₄
Al₂(SO₄)₃ + 3Ca(OH)₂ → 2Al(OH)₃ + 3CaSO₄