The earliest model of the atom.
What is the solid sphere model? (Sphere model acceptable.)
The first subatomic particle to be discovered.
What is the electron?
The particle(s) found in the nucleus.
Protons and Neutrons
The type of material that can block beta particles.
What is paper?
The phrase that describes an atom that has an electron in a higher energy level than normal.
What is "excited state"?
Ernest Rutherford discovered this model of the atom.
What is the nuclear model?
The second subatomic particle to be discovered.
What is the proton?
Number of protons:
Magnesium-25+2
What is 12 protons?
Lead blocks this type of radiation.
What is gamma radiation?
The type of radiation with a longer wavelength:
Infrared or Microwave
What is microwave?
Name this model:
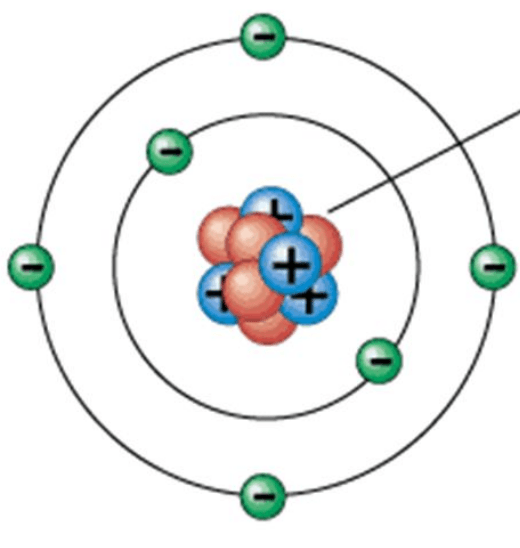
What is the planetary model? (Will accept Bohr model for 150 points.)
The scientist who discovered the electron.
Who is J. J. Thomson?
Number of electrons:
2760Co
What is 27 electrons?
The nuclear particle that consists of a helium nucleus.
What is alpha particle?
This color of the rainbow has the highest amount of energy.
What is violet? (Purple also acceptable.)
Name of this model:
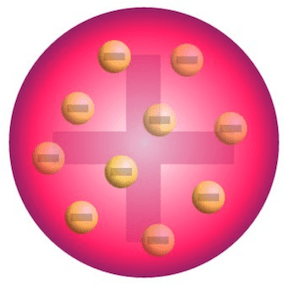
What is the plum pudding model?
The scientist who discovered the neutron.
Who is James Chadwick?
Number of neutrons:
Se-80
What is 46 neutrons?
Balance the nuclear reaction:
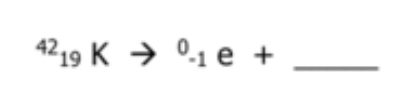
What is 1842Ar ?
Describe what must occur for an electron to jump to a higher energy level and what happens after.
An electron must absorb energy to jump to a higher level and it will eventually fall back down, releasing light in the process.
The most current model of the atom - name and who discovered it.
What is the quantum mechanical model discovered by Erwin Schrödinger?
The reason the electron was discovered.
What is because it has a negative charge and therefore can be deflected or attracted?
Number of electrons:
3888Sr+2
What is 36 electrons?
Balance the nuclear reaction:
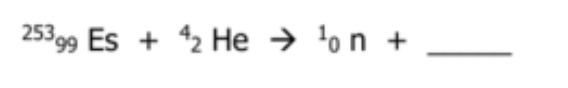
What is 101256Md ?
What radiation is most likely associated with the Balmer series - Visible, Ultra Violet, or Infrared?
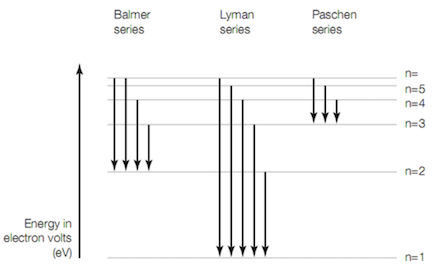
What is visible light?