_____________ (increasing / decreasing) will increase the number of collisions between molecules and ____________ (increase / decrease) the rate of reaction.
increasing, increase
Identify the activated complex.
C
_____________ are catalysts found in living organisms that increase the rate of reactions in cells.
enzymes
Combustion reactions are known to release energy in the form of heat and light. What will the enthalpy value be?
negative
What is the potential energy of the products?
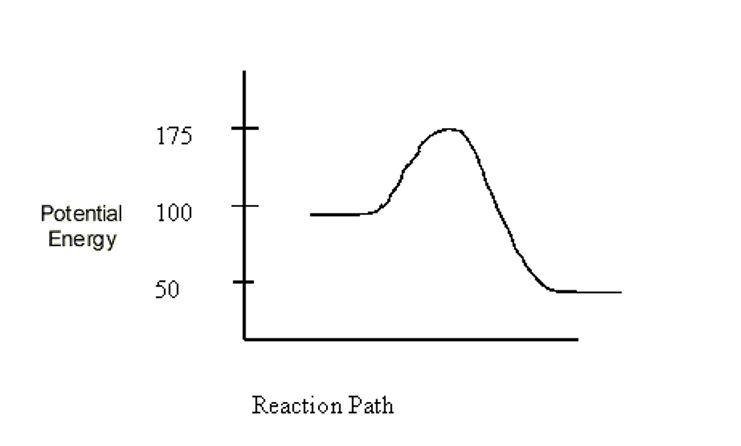
50kJ
In reactions, the molecules must __________ (collide/move past) to break the chemical bonds.
Collide
Is the reaction shown exothermic or endothermic?

endothermic
The forward reaction moves to the ___________ (left / right), while the reverse reaction moves to the (left / right).
right, left
Which bond is the strongest in the chart?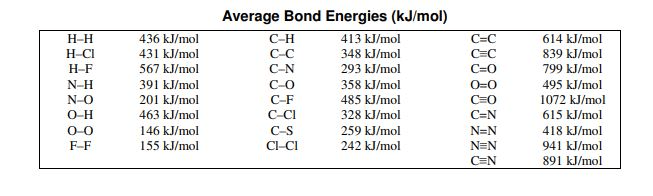
1072 Carbon Monoxide
Object A has a temperature of 50.0 0C, and object B has a temperature of 40.0 0C. When these objects are brought into contact, what will eventually occur?
Both objects will eventually be the same temperature
Chemical ___________ involves the study of reaction rates and pathways.
A. kinetics
B. equilibrium
C. collision theory
D. mechanisms
A. kinetics
What is B in the diagram below?

activation energy
A ________ _________ occurs when a reaction mechanism creates highly reactive elements that propagate the reaction, like knocking down a series of dominos.
Which of these bonds would release the least 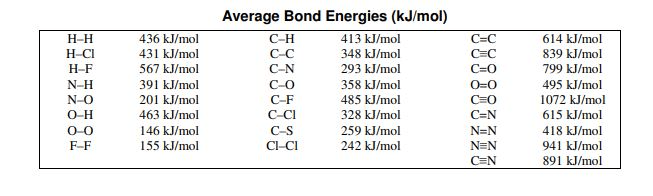 energy when formed?
energy when formed?
146 O-O
Which vessel will have the slowest reaction?
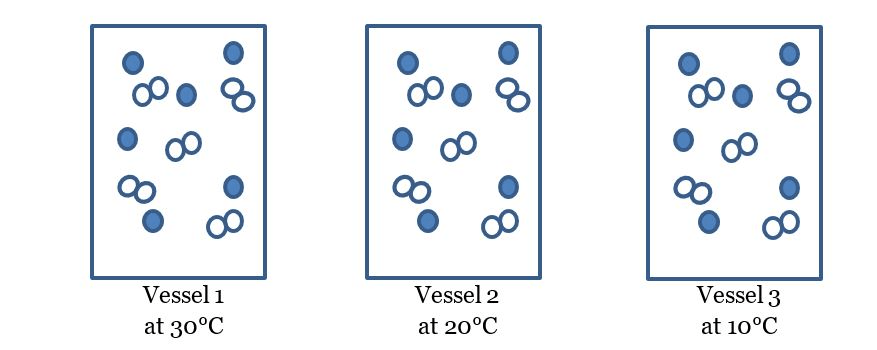
vessel 3
Name four ways to increase the rate of a reaction.
increase temperature, increase concentration, increase surface area, add a catalyst
Which shows the net change in energy for the reaction, Δ H or enthalpy?
D
Which of the following reactions would be more likely to occur in a single step?
A. H2 (g) + I2 (g) ⇌ 2 HI (g)
B. 2 C2H11 (l) + 15O2 (g) → 12 CO2 (g) + 6 H2O
A
Decomposition reactions often require heating for the reaction to occur. What can be said about the enthalpy of such reactions?
It is + and endothermic
Is the amount of energy the same, more, or less in breaking and forming chemical bonds?
same
How does decreasing the volume of a gaseous system affect the rate of reaction?
increases rate of reaction
What effect is demonstrated in the diagram below?

Which of the following diagrams will the reaction occur the quickest?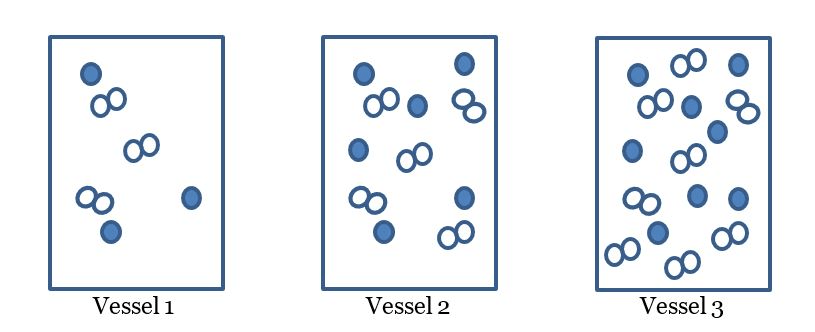
Calculate the total bond energy for the reaction:
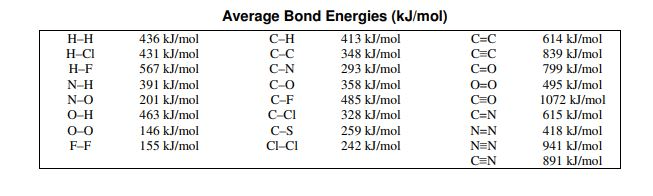 H2 + Cl2 -> 2HCl
H2 + Cl2 -> 2HCl
-184 kJ
Which vessel has the greatest concentration?
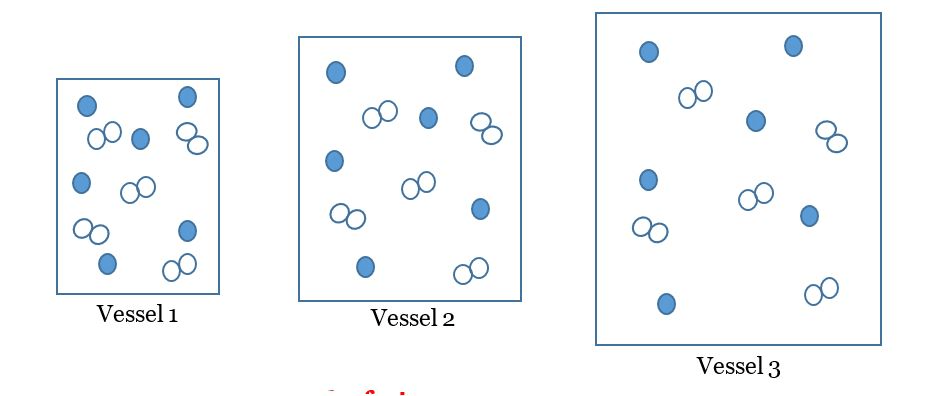
vessel 1