Which subatomic particle has no charge?
Neutron
An atom has 73 protons. What element is it?
Tantalum.
This is an atom of...
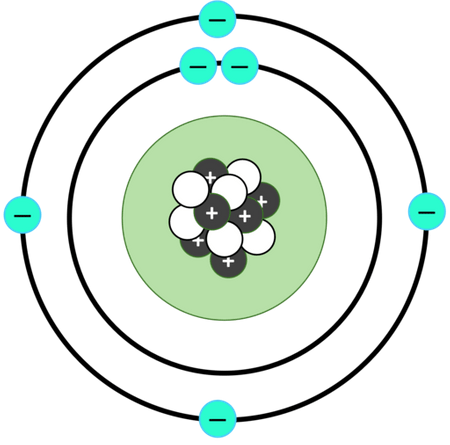
Carbon (6 protons, neutrons, and electrons)
Say the law of conservation
Matter cannot be created nor destroyed, only rearranged.
The saying to remember which side the reactants are on in a chemical reaction is ...
"Reactants really come first"
Energy moves from one organism to another through
Can forces act on you without touching you? Give an example.
Yes! Gravity/ Magnets
Name all 3 subatomic particles
Protons, neutrons, electrons
You have an atom with 24 protons. A scientist removed 5 of them. What element did it turn into?
Potassium
24-5 = 19 (atomic number)
Nitrogen is red, Hydrogen is Grey. What is the chemical formula of this model?
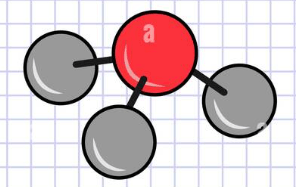
NH4
If you start with 12g of Oxygen and react it with 8g of Hydrogen, what is the mass of the products?
20 grams
How many atoms are on the reactant side?
C₂H₆ + O₂ → CO₂ + H₂O
10 atoms.
A food chain ALWAYS begins with a ..
Producer (plants)
What is responsible for building mountains, subduction zones, and mid Ocean ridges, constantly shaping and changing the Earth (slowly)
Tectonic Plates
An experiment started with 23 grams of reactant but ended with 19 grams of product. Explain what could have caused this to happen.
They may have used an open system, causing some matter to escape.
What is the atomic mass of Iodine?
Around 126.9
Does this follow the law of conservation? Explain it.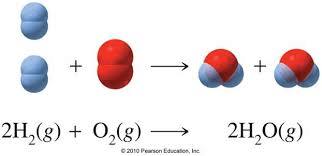
Yes. 4 hydrogen and 2 oxygen in reactants, the same after it reacts in the products.
Does this follow the law of conservation?
CH₄ + O₂ → CO₂ + H₂O
No. Reactants have 1 carbon, 4 hydrogen, 2 oxygen. Products have 1 carbon, 2 hydrogen, 3 oxygen. THEY DO NOT MATCH.
Name this chemical reaction: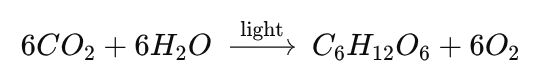
Photosynthesis
Say the 1st Newton's Law
An object in motion stays in motion, an object at rest stays at rest, unless acted upon by an unbalanced force.
There's a mountain with 10 different layers. The top rock is 80,000 years old. What is the RELATIVE age of the rocks below it?
OLDER than 80,000 years.
Explain why a closed system is SO MUCH BETTER than an open system.
Closed = no matter can escape = more accurate data
Why is an atom of Hydrogen different than at atom of Helium? (They're made of the same thing??)
They have different amounts of subatomic particles, which makes them different elements.
A student made this model of CH4. Explain why it is wrong.
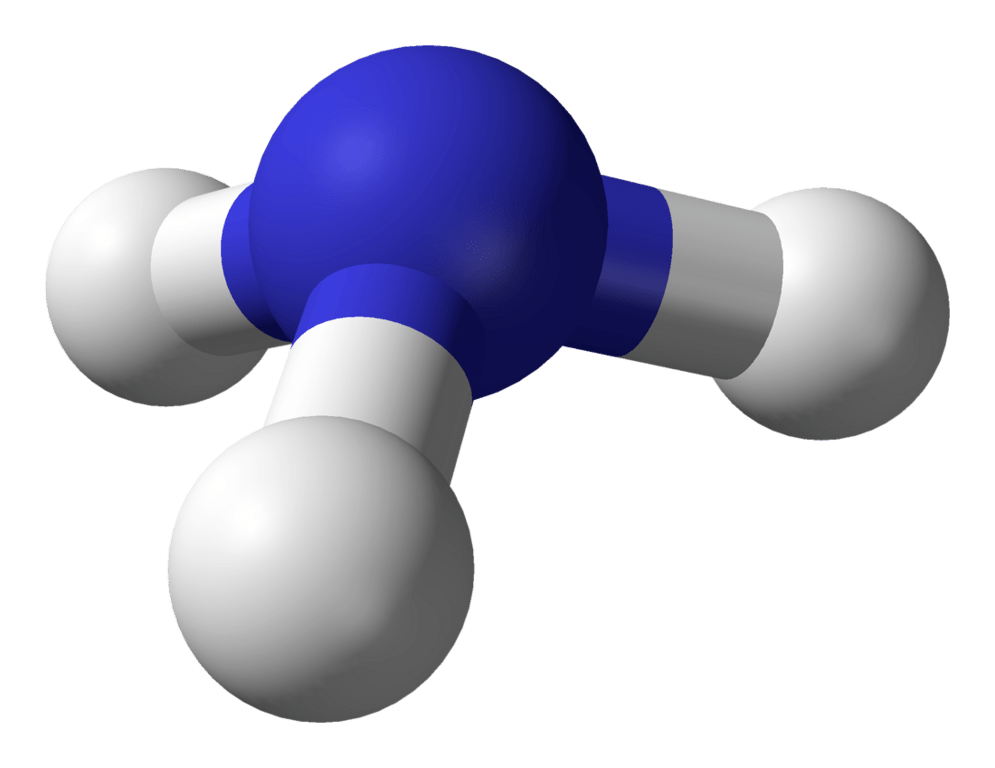
They're missing a hydrogen.
Does this follow the law of conservation?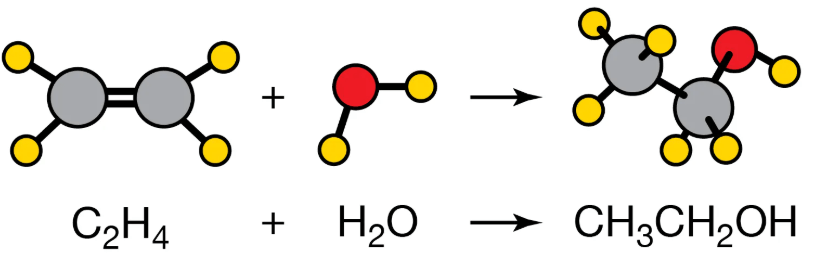
Yes, both the reactants and products have the same amount of each atom.
How many molecules are on the reactant and product side? Does this determine if it follows the law?
4Fe + 3O₂ → 2Fe₂O₃
6 on Reactant, 2 on Product. Molecules do NOT determine if the law is followed. Only atoms.
Name 7 organelles
TBD
Which sound wave would be louder and WHY?
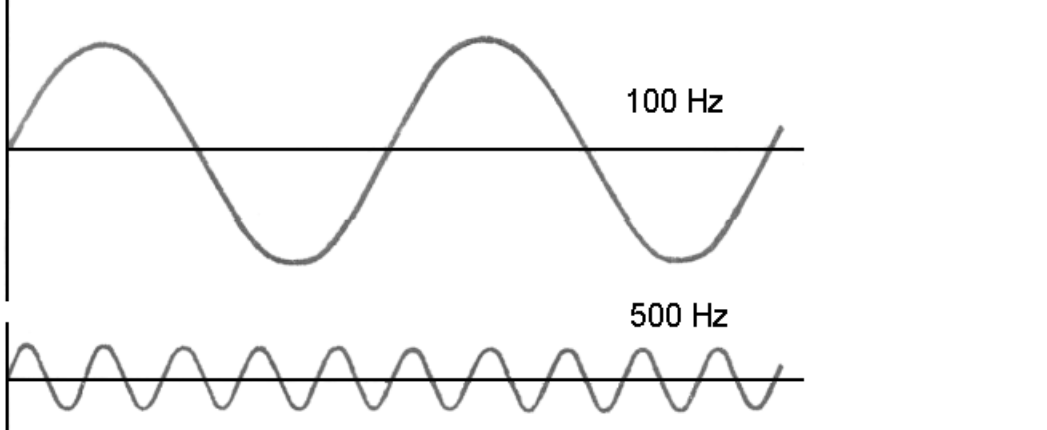
The top one, its waves are taller. Which means it has a larger amplitude.
What's the chemical formula for sugar? (Glucose)
C6H12O6
Which element, from the fact of the day 3 weeks ago, was MAN MADE? (Hint: it's a big one)
Oganesson
When making a model, how many bonds can each of these hold:
Carbon
Oxygen
Hydrogen
Carbon: 4
Oxygen: 2
Hydrogen: 1
2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
How many molecules are on the products side?
18 molecules.
How many atoms are in the reactants AND products of this chemical reactions?
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Both sides have 30 atoms.
Which are bacteria, plant, and animal cells. EXPLAIN.
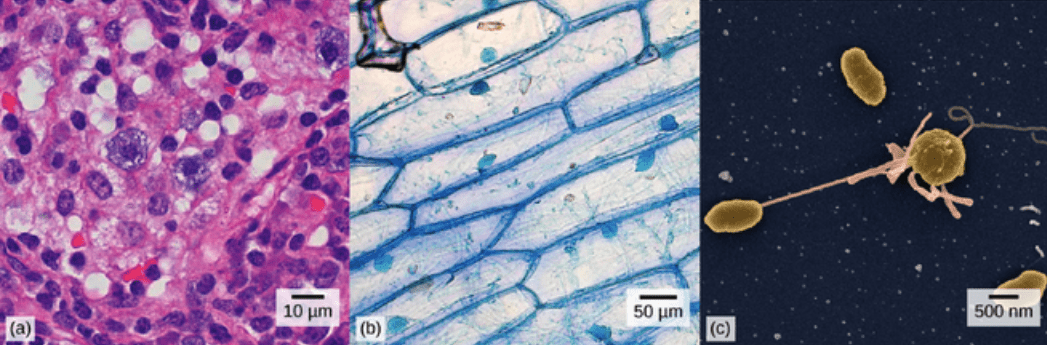
Animal, plant, bacteria.
What are the TWO causes the seasons?
The tilt of the Earth's axis and it's position around the Sun.