Gold, solid sodium chloride, water, mercury are examples of _______
Pure substances
I discovered the positively charged nucleus of the atom
Rutherford
I am an element in group 2, period 6, what am I ?
barium
What is an exothermic reaction?
Give an example
Heat is released into the environment.
Example (wood burning)
a) Name a compound that tastes sour and is binary?
b) Name a compound that tastes bitter
HCl
NaOH
37 -17= 20
(mass number - proton #)= number of neutrons
I discovered the tiny indivisible particles
Dalton
Name another element that has similar chemical properties to chlorine?
halogens (bromine, iodine, fluorine, et in the same group)
5 Signs of chemical reaction?
Colour change, odour change, formation of gas, formation of a solid precipitate, temperature change
Name a compound that is ionic and does not conduct electricity?
AgI (s)
CuI (s)
An element with the same number of protons
different number of neutrons and mass number
Chocolate chip cookie model
Thomson
What type (A/B/I/M) is N5 H 7
Name it?
Molecular
pentanitrogen heptahydride
What does law of conservation of mass state?
Mass is not destroyed or created in a chemical reaction it is only converted.
Name a compound that is molecular and neutral (not acid or base)
glucose
sucrose
water
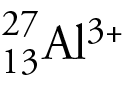
Has how many protons, electrons and neutrons?
protons: 13
neutrons: (27-13= 14)
electrons: 10
The most current model : the electron cloud is a particular area in which an electron is likely to be.
Quantum model of atom
What type of compound is Po(SO4)2
Name that?
Ionic
polonium (IV) sulfate
C4H10 is burned in oxygen, write the balanced equation and identify type of reaction
hydrocarbon combustion
a compound was discovered to react with metal to produce a gas. This compound contains carbon. What can it be?
organic acid (HCOOH) or others
Name an ion that has the same number of electrons as neon
Mg2+
F-
O2-
How many valence electrons are there in a fluoride ion
8
Name H2S vs H2SO4
What is the difference?
hydrosulfuric acid vs sulfuric acid
binary acid vs oxyacid
Give 3.5 g of water. Calculate the number of atoms of hydrogen?
2.338...x1023 atoms of hydrogen
make a double replacement reaction that produces a precipitate
variety of answers