When you make a hot chocolate, this is the solvent.
Milk (or water)
How do you calculate the concentration of a solution? Write the equation.
Concentration = moles/volume
What is the equation that relates moles and mass of a substance?
Moles = mass/molar mass
Write the dissociation equations for calcium nitrate
Ca(NO3)2(s)--> Ca2+(aq) + 2NO3-(aq)
Balance this equation:
C3H8 + O2 → CO2 + H2O
C3H8 + 5 O2 → 3CO2 + 4H2O
This type of reaction is produced when 2 solutions is reacted to produce a insoluble solid.
What is Precipitation reaction
When you’ve got a 10g solution of NaCl, and 2.5g of it is NaCl, what’s the percent mass of the solution?
Must show work and use correct units for points.
25%
How many grams are in 88.1 moles of magnesium?
Must show work and use correct units for points.
2114.4 grams
Draw 4,5-dimethyl-hexan-3-ol
DAILY DOUBLE!!!
Wager an amount, based on how many points you have. If you get it right, you get that amount. If you get it wrong, we subtract the amount from your score.
Name this compound: Cr3(PO4)2
Why was hexane able to dissolve oil, but water wasn’t?
Acetone is nonpolar and oil is nonpolar.
Water is polar.
Like dissolves like.
Determine the concentration of the solution with 0.4 mol of NaOH in 100 mL total volume of solution.
Must show work and use correct units for points.
100mL = 0.1L
C = 0.4 / 0.1 L= 4 mol/ L
How many moles are in 22g of Argon, Ar?
Must show work and use correct units for points.
0.55 mol
Describe the kind of molecules that can undergo cracking. What is the purpose of cracking?
Long chain alkanes.
Purpose of cracking is to produce shorter chain alkanes which are more in demand (and to produce smaller alkenes which can be used in polymerization).
Name this guy:
Dimitri Mendeleev
Describe what “saturated solution” means.
A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent.
DAILY DOUBLE!!!
Only one team answers.
Wager an amount, based on how many points you have. If you get it right, you get that amount. If you get it wrong, we subtract the amount from your score.
What is the concentration of a solution that has 0.475 g of magnesium chloride (MgCl2) dissolved in 100 cm3 of water?
What mass of carbon dioxide is produced when 0.250L of a 0.2 molL-1 solution of sulfuric acid is added to excess sodium carbonate?
Must show work and use correct units for points.
2.20 g CO2
This is the monomer.
Draw the repeating unit of the polymer and name the polymer.
Name: polypropene
Repeating unit: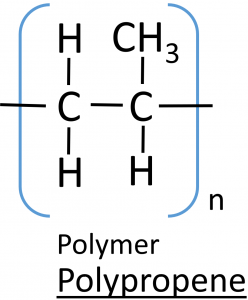
Name the largest element formed by in a star.
Iron (Fe)
Describe the difference between how ionic compounds are dissolved in water and how covalent compounds are dissolved in water.
Ionic - water surrounds each ion to separate it from the lattice structure
Covalent - water surrounds each covalent molecule compound
Describe how to make a 500mL of a 2.0M calcium nitrate solution.
No calculations necessary, but equations used should be written.
1. Determine how many moles of calcium nitrate is needed (n = C x V)
2. Calculate how many grams of calcium nitrate that is. (m = moles/molar mass)
3. Weigh out that amount of calcium nitrate.
4. Measure out 500mL of water
5. Dissolve the calcium nitrate in the water.
Using the balanced equation below:
N2 + 3H2 → 2NH3
Calculate the theoretical yield of NH3 in grams if you had 8 mol of hydrogen gas. Must show work and use correct units for points.
90.6g NH3
Draw all of the isomers of C4H9Br.
Name them.
Marie Curie, who the element Curium was named after, got into a bit of a scandal when she got romantically entangled with her late husband’s former student, Paul Langevin (he was married!!)
At the height of this scandal, she accomplished something major. What was it?
She won her second(!!!) Nobel Prize