The majority of the mass of an atom is in this part of the atom.
What is the nucleus?
Number of significant figures in 10.83 m.
What is 4 sig figs?
H2 + O2 --> H2O
What is 2H2 + O2 --> 2H2O?
2KI + MgCl2 --> 2KCl + MgI2
1 mol of KI and plenty of MgCl2 will make this amount of MgI2 (mol).
What is 0.5 mol of MgI2?
Notation for alpha particle
What is 42He?
The subatomic particle that has a charge of -1.
What is an electron?
Number of significant figures in 0.021 seconds.
What is 2 sig figs?
NaF + MgCl2 --> NaCl + MgF2
What is 2NaF + MgCl2 --> 2NaCl + MgF2?
2KI + MgCl2 --> 2KCl + MgI2
76.4g of MgCl2 & plenty of KI will make this amount of KCl (mol).
What is 1.60 mol of KCl?
What needs to be balanced in a nuclear reaction?
What are atomic number and mass number?
The subatomic particle that determines what element an atom is.
What is the proton?
(5.79 m + 42.6 m) / 7.230 s
What is 6.69 m/s?
H3PO4 + KOH --> K3PO4 + H2O
What is H3PO4 + 3KOH --> K3PO4 + 3H2O?
2KI + MgCl2 --> 2KCl + MgI2
76.4g of MgCl2 & plenty of KI will make this amount of MgI2 (grams).
What is 223 grams of MgI2?
Iron-55 decays through electron capture. Write the nuclear reaction.
What is 5526Fe +-1e --> 5525Mn?
Selenium-77 has this number of neutrons.
What is 43 neutrons?
Joe completed the marathon in 180 minutes. How many seconds did Joe take?
What is 11000 seconds?
Ca(ClO3)2 + KI --> CaI2 + KClO3
What is Ca(ClO3)2 + 2KI --> CaI2 + 2KClO3?
2KI + MgCl2 --> 2KCl + MgI2
500g of KI & 800g of MgCl2 react. Identify the limiting reactant.
What is KI?
Carbon-14 decays through beta particle emission. Write the nuclear reaction.
What is 146C --> -1e + 147N?
Iodine-127 with a net charge of -1 has this number of electrons.
What is 54 electrons?
Measure the amount of water in the graduated cylinder (mL):
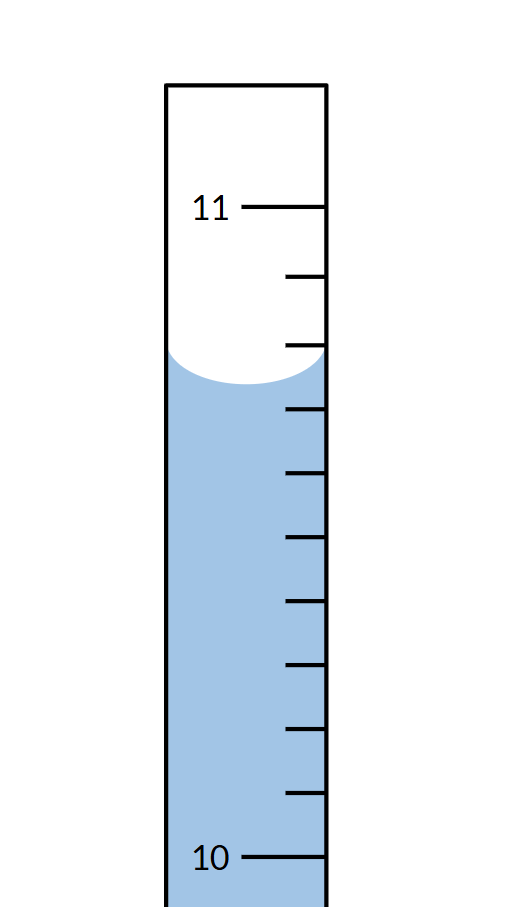
What is 10.73 mL?
C5H12 + O2 --> CO2 + H2O
What is C5H12 + 8O2 --> 5CO2 + 6H2O?
2KI + MgCl2 --> 2KCl + MgI2
300.g of KI & 150.g of MgCl2 react. Determine amount of KCl made (grams).
What is 134g of KCl?
23592U +10n --> 8735Br + ____ + 310n
What is 14657La?