This is the number of significant figures in 0.0009
What is 1?
These are two possibilities of units for the Avogadro's number.
What are atoms/, ions/, molecules/, particles/, electrons/, photons/, formula units/mole?
The SI unit of energy is derived from these base units.
What is kg*m2/s2 ?
The name of the second group on the periodic table.
What is the alkaline earth metal group?
Here's mine: Raging Martians Invade Venus Using X-Ray Guns
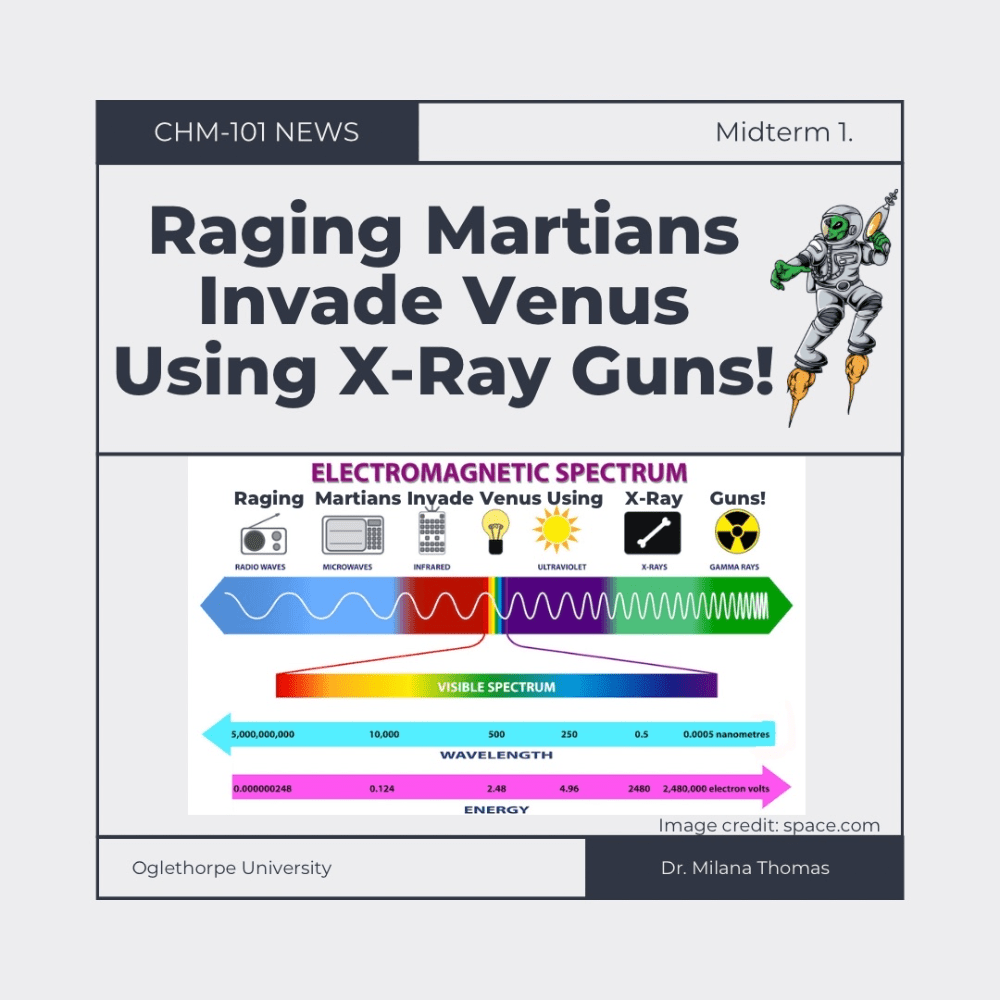
The name of three SI units.
What are (1) second, s, for time, (2) meter, m, for length, (3) kilogram, kg, for mass, (4) ampere, A, for electric current, (5) kelvin, K, for thermodynamic temperature, (6) mole, mol, for amount of substance, and (7) candela, cd, for luminous intensity.
The weight of one mole of iron.
What is 55.845 g?
What is wavelength?
This is the only element in its group that is not an alkali metal.
What is hydrogen?
Why?
X-ray.
X-ray radiation has higher energy.
This is the number of kg in 2.525 mg
What is 2.525 x 10-6 kg?
The number of moles of Ca in 1.927 x 1024 atoms of calcium.
What is 3.200 moles?
Convert 1.87*1019 eV to Joules.
Hint: 1.602 x 10-19J = 1 eV
What is 3 Joules
The name for type A elements.
What are main group elements?
How are energy and frequency related? Which equation tells you this?
Directly proportional. E=hv
The number 003903000 in scientific notation.
What is 3.903*106?
This is the number of H2O molecules in a snowflake weighing 1 mg.
Hint: The mass of H2O is 18.02 g/mol
What is 3 x 1019 H2O molecules?
This is the frequency of a photon that has a wavelength of 420 nm.
What is 7.1x1014Hz?
(units could also be "1/s" or "s-1)
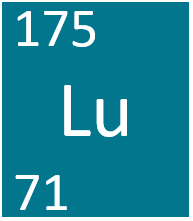
This is the isotopic symbol for an element with 71 protons and 104 neutrons.
How are energy and wavelength related? How do you know.
Inversely proportional. Wavelength and frequency are inversely proportional.
____ cm3 of water in 30 mL of water.
(hint: 1000 L = 1 m3)
What is 30 (cm3 of water)?
The number of moles of copper in a cube of copper metal with an edge length of 1.00 in.
Hint: 1 in = 2.54 cm, density of copper= 8.92 g/cm3
What is 2.30 moles of copper?
Bonus (because generally, I would just tell you that this is the frequency): The energy of a photon whose wave peaks pass a given point every 8.00 x 1014 s.
What is 5.30 x 10-19 J?
Bonus question!!
This is the name of the periodic table group that has the property of fluorescence.
What is the noble gas group?
How do you find wavelength if you know energy?
E=hc/wavelength