This element’s name sounds like it belongs in Santa’s workshop: Fe.
Iron
Safety equipment you should wear when doing holiday-themed experiments.
Goggles
The subatomic particle that determines an atom’s chemical behavior.
This element, symbol He, is used to fill balloons for holiday parties.
Helium
The number of protons in an atom, which you can find at the top of each element’s box on the periodic table.
Atomic number
This noble gas is often used in bright, colorful holiday signs.
Neon (Ne)
Dry ice is actually solid __________.
Carbon dioxide (CO2)
The region around the nucleus where electrons are most likely found.
Electron Cloud
(I will also accept rings!)
Elements in the same column, like Na and K, are called this and have similar properties.
Groups or families
This tells you the total number of protons and neutrons in an atom and is often listed as a whole number on the periodic table.
Atomic mass AKA mass number
This element is a main component of coal, often linked with winter heating.
Carbon (C)
This tool is used to accurately measure liquids in a lab.
Atoms of the same element with different numbers of neutrons are called these.
Isotopes
Shiny metals like Al (used in tinsel) and Fe (in decorations) belong to this category.
Metals
Draw the Bohr model for magnesium (Mg).
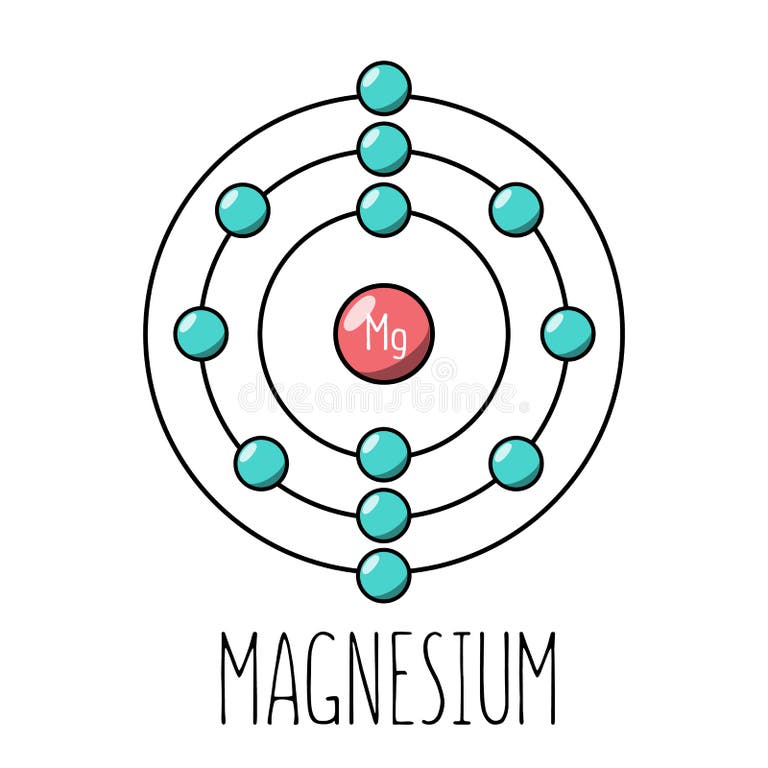
The element used in tinsel because it’s shiny and lightweight.
Aluminium (Al)
This piece of lab equipment is used to safely heat substances, like warming water for a winter experiment. (Hint: Think Breaking Bad!)
Bunsen burner
The maximum number of electrons that can fit in the first energy level of an atom.
2
This element, symbol Ag, is often associated with silver decorations and ornaments on a Christmas tree.
Silver
Draw the Bohr model for a chlorine ion (Cl⁻). How does the number of electrons differ from the neutral atom?
(Need to see both the drawing and answer to the question!)
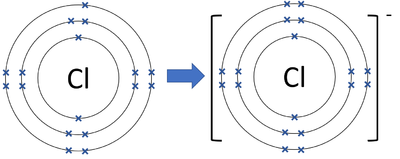
The Cl- ion has an extra electron making the 3rd ring have 8 electrons instead of 7.
This radioactive element is sometimes used in small power sources for space probes launched in winter.
Plutonium
The scientific study of matter and its changes—perfect for understanding holiday science.
Chemistry
This term describes an atom that has gained or lost electrons and therefore has a charge.
Ion
This element, symbol Tl, is a post-transition metal that can produce a faint green color in certain chemical reactions and has historically been used in small holiday pyrotechnics.
Thallium
Draw the Bohr model for phosphorus ion (P³⁻). Show all electrons in their correct energy levels, including the extra electrons from the ion.
(The dots are the extra electrons for the ion state)