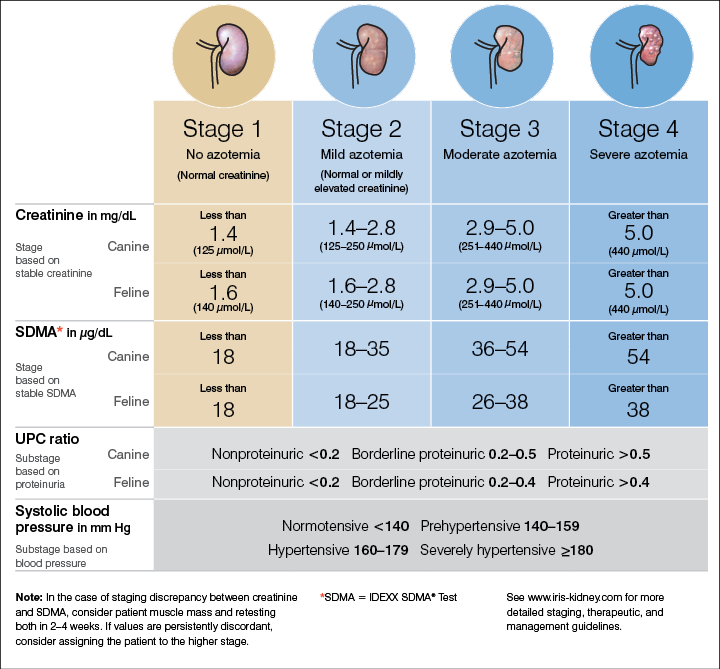
A cat has creatinine 2.6 mg/dl, UPC 0.3, and SBP 145 mmHg. Stage this patient fully.
What is IRIS Stage II, borderline proteinuric, prehypertensive?
The most common morphologic lesion in cats with CKD.
What is lymphoplasmacytic tubulointerstitial nephritis with fibrosis?
Can also see tubular degeneration + atrophy, mineralization of Bowman's capsule + tubular basement membranes, and glomerulosclerosis.
Why anemia develops in CKD
What is decreased EPO production, shortened RBC lifespan, and chronic inflammation/iron sequestration/iron deficiency?
This test can help distinguish CKD from AKI when historical information is unclear.
What is renal ultrasound (small irregular kidneys = CKD, large painful = AKI)?
The important dietary modification proven to slow CKD progression.
What is phosphorus restriction?
True/False — CKD is generally reversible with appropriate therapy.
What is False?
The major limitation of creatinine in staging CKD.
What is its dependence on muscle mass, hydration status, and laboratory variability?
Creatinine has limitations in staging CKD because it’s an indirect marker of GFR and is affected by factors other than kidney function. Muscle mass, diet, and hydration status all influence creatinine: animals with low muscle mass (like older or cachectic cats) may have “normal” creatinine despite significant renal damage, while dehydration can falsely elevate it. Also, creatinine doesn’t rise until roughly 65–70% of nephrons are lost, so it misses early disease. This is why IRIS incorporates SDMA, proteinuria, and blood pressure to improve accuracy beyond creatinine alone.
Different laboratories (and even different analyzers within the same lab) may use slightly different assays, reference intervals, and calibrations, which can cause small but clinically meaningful shifts in creatinine results. For example, a creatinine of 2.0 mg/dL at one lab might fall in a different IRIS stage if measured at another. This is why serial trends in the same patient, ideally measured at the same laboratory, are more reliable than single isolated values.
Breed-specific nephropathy: Amyloidosis is strongly associated with this dog breed and this cat breed.
What are Shar Pei dogs and Abyssinian cats?
Also English Foxhound and Beagles
The pathophysiologic reason cats often develop hypokalemia with CKD.
What is renal potassium wasting due to impaired tubular reabsorption?
Why ionized calcium should be prioritized over total calcium in CKD.
What is altered protein binding and acid–base status make total calcium misleading?
In CKD, ionized calcium (iCa) should be prioritized over total calcium because iCa is the biologically active form. Total calcium is influenced by protein binding (especially albumin) and complexation with phosphate, both of which are frequently abnormal in CKD. For example, a patient with hypoalbuminemia may have a low total calcium but normal ionized calcium, while hyperphosphatemia in CKD can bind calcium and lower total calcium without necessarily changing the active fraction. Because CKD disrupts acid–base balance, phosphate handling, and protein levels, total calcium is an unreliable indicator, whereas iCa gives the true picture of the patient’s calcium status and guides management of mineral and bone disorders
The rationale for darbepoetin over epoetin in veterinary CKD anemia.
What is longer half-life and reduced antibody formation risk?
Prognostic factors positively associated with mortality in CKD.
What are hyperphosphatemia, hypoalbuminemia, hypertension, and high UPC ratio?
This biomarker has been integrated into IRIS guidelines to help detect CKD earlier than creatinine.
What is SDMA (symmetric dimethylarginine)?
The variation in histopathological location of lesions between dogs and cats.
What is species-specific variation in histopathologic lesions - dogs favor glomerular + cats tubulointerstitial?
Explain why hyperphosphatemia worsens CKD progression.
What is secondary hyperparathyroidism causing nephrocalcinosis, mineralization, and further nephron loss?
In CKD, fewer nephrons can excrete phosphate, leading to phosphate retention.
High phosphate stimulates fibroblast growth factor-23 (FGF-23) and parathyroid hormone (PTH) release → secondary renal hyperparathyroidism.
Excess PTH and FGF-23 drive bone resorption and disturb mineral metabolism.
High calcium–phosphate product causes soft tissue and renal mineralization (nephrocalcinosis), which directly damages tubules and interstitium.
Phosphate also promotes tubulointerstitial fibrosis by stimulating pro-inflammatory and pro-fibrotic pathways.
The prognostic significance of a urine protein-to-creatinine (UPC) ratio ≥2.0 in a CKD dog.
What is strong association with glomerular disease and poorer survival outcomes? Can also aid in determining microanatomic location of the lesion. The severity of the increase in UPC is proportionate to the likelihood of primary glomerular disease.
Why is aggressive fluid diuresis not appropriate in decompensated CKD?
What is risk of overhydration, electrolyte derangements, and no restoration of lost nephrons?
Goal is not to induce diuresis - already have a diuresis due to impaired renal concentrating ability.
Goals = normalize hydration status & improve acid-base/electrolyte abnormalities.
Prognostic factors negatively associated with mortality in CKD (protective).
What are good body condition score and preserved muscle condition?
A dog has creatinine 5.2 mg/dl, UPC 0.7, SBP 182 mmHg. Which substages indicate the highest risk of target organ damage, and why?
What are proteinuric and severely hypertensive, because both accelerate renal injury and systemic complications?
A juvenile Soft-Coated Wheaten Terrier presents with proteinuria and azotemia. What congenital nephropathy is most likely?
What is primary glomerulopathy?
Also renal dysplasia
Why advanced CKD patients may present with seizures or altered mentation.
What is uremic encephalopathy due to neurotoxic metabolites crossing the blood–brain barrier?
As kidney function declines, uremic toxins such as guanidino compounds, organic acids, and other nitrogenous wastes accumulate in the bloodstream and cross the blood–brain barrier. These toxins disrupt neurotransmission, alter neuronal excitability, and can cause cerebral edema or vascular injury. The result is a spectrum of neurologic signs, from dullness and disorientation to tremors, seizures, and even coma.
Renal biopsy has this major potential risk in CKD.
What is bleeding secondary to uremic inhibition of platelet function?
Why mirtazapine dosing must be reduced in cats with CKD.
What is prolonged half-life due to reduced renal clearance?
Mirtazapine stimulates appetite and reduces nausea by blocking presynaptic α₂-adrenergic and serotonin (5-HT₂/5-HT₃) receptors, increasing norepinephrine and serotonin release while also having mild sedative effects via H₁ blockade
Why renal transplantation is limited in veterinary medicine.
What is cost, availability, immunosuppression risk, and limited donor pools (esp. in dogs)?
Why patients staged as IRIS I (normal creatinine) can still have significant CKD.
What is early CKD with loss of renal reserve, masked by creatinine’s insensitivity to mild reductions in GFR?
The mechanistic link between interstitial fibrosis and progressive nephron loss.
What is tubulointerstitial inflammation leading to hypoxia, capillary rarefaction, and further tubular atrophy?
Interstitial fibrosis compresses peritubular capillaries → tissue hypoxia → tubular atrophy → nephron dropout. Creates a vicious cycle of progressive loss.
A CKD patient presents with weight loss, anemia, and normal creatinine. What explains the discrepancy?
What is muscle wasting lowering creatinine despite significant renal dysfunction?
What it is: Creatinine is a breakdown product of creatine in muscle, produced at a relatively constant rate and excreted by the kidneys.
Why it’s used: Because it’s freely filtered by the glomerulus, serum creatinine is used as a surrogate marker of glomerular filtration rate (GFR).
Limitations in CKD:
Insensitive to early disease – Creatinine only rises when ~65–70% of nephrons are lost, so mild CKD may be missed.
Influenced by muscle mass – Cachectic or older animals may have “normal” creatinine despite CKD, while very muscular animals may look falsely high.
Affected by hydration – Dehydration can transiently increase creatinine; overhydration can lower it.
Lab variability – Differences in assays and reference ranges mean values should be compared within the same lab.
A CKD cat’s bloodwork shows azotemia, metabolic acidosis, and hypokalemia. Which abnormalities most directly predispose to muscle weakness, and why?
Hypokalemia causes muscle weakness because low potassium makes muscle cells hyperpolarized, so they’re less excitable and can’t contract effectively, while metabolic acidosis further impairs muscle function by interfering with contractility and promoting protein breakdown; together, they make CKD patients prone to weakness and fatigue, with cats sometimes showing cervical ventroflexion as a classic sign.
Hypokalemia: With less extracellular K⁺, the gradient for K⁺ efflux increases. More K⁺ leaves the cell, making the RMP more negative (hyperpolarized).
Effect: Because the cell is further from threshold, it becomes harder to depolarize and fire an action potential.
A CKD patient is hypertensive. What is your pharmacologic next step?
Calcium channel blocker - amlodipine
Angiotension converting enzyme inhibitor - enalapril or benazapril
Angiotensin receptor blocker - telmisartan
Compare the prognosis of cats with IRIS Stage 1–2 vs. Stage 3–4 CKD.
What is significantly longer survival (years vs. months–1 year), with stage 3–4 showing rapid decline post-diagnosis?