Element or Compound: O3
What is an element?
A physical combination of two or more substances that aren't chemically joined - e.g., saltwater.
What is a mixture?
The type of mixture that can only have one state of matter.
What is a homogeneous mixture?
Although on the left side of the periodic table, this element is not a metal, and is in fact a nonmetal.
What is Hydrogen?
Boiling point and melting point.
What is a physical property?
Element or Compound: C6H12O6
What is a compound?
A homogeneous mixture where the dissolved particles are less than 1nm.
What is a solution?
True or False: A compound displays the properties of the elements that make that compound.
What is false?
These elements are brittle semiconductors of electricity.
What are metalloids?
Flammability (the likelihood something will burn).
What is a chemical property?
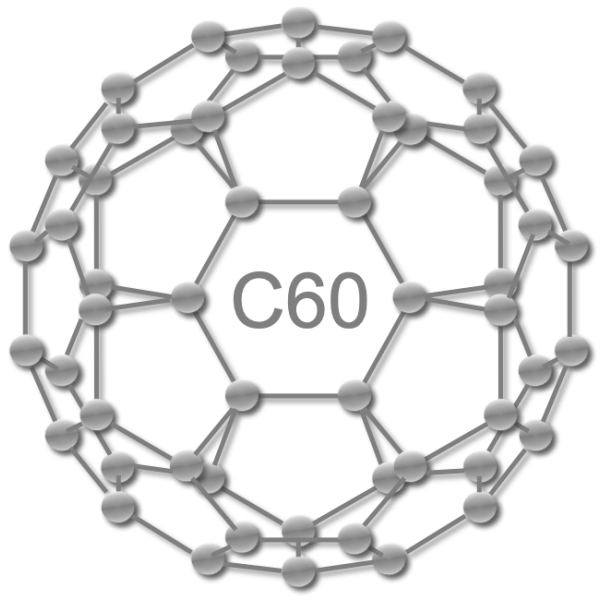
Element or compound?
What is an element?
A uniform mixture.
What is homogeneous?
Colloids and suspensions.
What are heterogeneous mixtures?
These elements are the best conductors of heat.
What are metals?
Rocks being worn away by the ocean waves.
What is a physical change?
The basic unit of matter.
What is an atom?
A mixture that cannot be filtered but whose particles display the Tyndall Effect.
What is a colloid?
Of the three types of mixtures, this one will settle and allow particles to be filtered.
What is a suspension?
These elements may or may not have luster.
What are metalloids?
Your sweat cooling your skin on a hot day.
What is a physical change?
Of S8 (Sulfur) or He (Helium), this one is both an element and a molecule.
What is S8?
Salad, trail mix and milk.
What is a heterogeneous mixture?
The smallest unit of a substance that retains the properties of that substance (e.g., C6H12O6).
What is a molecule?
Some of these elements are totally nonreactive.
What are nonmetals?
Your digestive system breaking down food.
What is a chemical change?