1) 3% solution of hydrogen peroxide
2) reese's peanut butter cup
2) What is a heterogeneous mixture?
Polar or nonpolar?
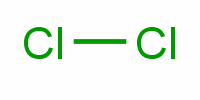
(A) Gasoline is dangerous because it easily evaporates to fill a given space with flammable vapors.
(B) Carbon monoxide kills by bonding irreversibly to red blood cells.
(C) Ink moves up the paper during TLC.
Na + Cl2 → NaCl
Polar or nonpolar?

carbon dioxide + carbon → carbon monoxide
Silver(I) fluoride + calcium chloride → silver(I) chloride + calcium fluoride
a) H2+
b) O2+
c) Mg2+
Polar or nonpolar?

HNO3 + Al(OH)3 → Al(NO3)3 + H2O
AlF3 + HgCl2 → AlCl3 + HgF2

copper + silver(I) chloride → copper(I) chloride + silver
HNO3 + Al(OH)3 → Al(NO3)3 + H2O
2 part answer!

lead(II) nitrate → lead(II) oxide + nitrogen dioxide + oxygen gas
aluminum(III) oxide → aluminum + oxygen