Define atom.
The smallest unit of matter.
Define matter.
Anything that has mass and takes up space.
When sodium bicarbonate reacts with acetic acid, carbon dioxide and water is produced. What is the total mass conserved during this reaction?
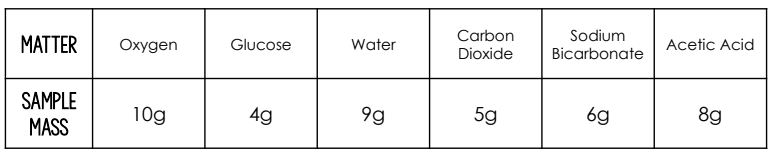
13 grams
During photosynthesis, oxygen and glucose are produced. What is the total mass of the matter transformed during this chemical reaction?
14 grams
During which chemical reaction is matter destroyed rather than transformed?
a. burning wood
b. digestion of food
c. photosynthesis
d. none of the above
None of the above
When chemical or physical reactions take place, the ______________ get rearranged but do not get created nor destroyed.
atoms
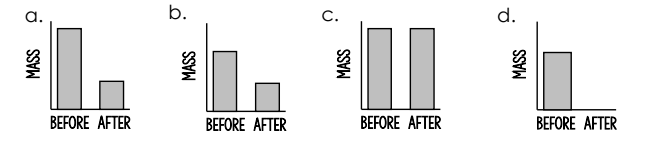
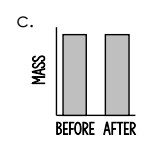
Which graph shows the amount of matter present before and after a chemical reaction between butyl alcohol and hydrogen peroxide in a glow stick?
During photosynthesis, what are the reactants?
(hint: What is the plant taking in?)
Carbon dioxide and water
Define physical change.
A change in the observable properties of matter.
State the Law of Conservation of Matter.
Matter cannot be created nor destroyed, only changed (transformed).
According to the Law of Conservation of Matter, the amount of ____________ must be the same in both the reactants and products of a reaction.
mass
During photosynthesis what are the products?
(hint: What does the plant give off?)
Oxygen and glucose
Define chemical change.
A change in chemical structure, resulting in a new substance.
Which of the following is true?
a. Matter is only conserved when substances undergo physical change.
b. Matter is conserved during chemical and physical changes.
c. Matter is only conserved during chemical changes.
d. Matter is only conserved when substances change state.
Matter is conserved during chemical and physical changes.
If 250 grams of white glue and 200 grams of dish soap react to form a new solid, what would you expect the mass of the solid to be?
450 grams
During photosynthesis, the mass of the carbon dioxide and water is ______________ to the mass of the glucose and oxygen.
equal
During physical and chemical changes, matter is _______________.
transformed
When matter is transformed during a chemical reaction, the mass is ______________.
conserved
If 15 grams of powder reacts with 12 grams of liquid to produce a bubbling black liquid with a mass of 22 grams, what can be concluded?
a. the reaction violates the law of conservation
b. 5 grams of gas was produced
c. 8 grams of gas was produced
d. the reaction isn't over
5 grams of gas was produced
Explain the process of photosynthesis.
In order to make energy for survival, a plant must use light energy to produce a reaction between carbon dioxide and water. Glucose and oxygen are produced as a result of the reaction.