What is the charge of a proton?
+1
Is fusion or fission more stable?
fusion
The spring sport Mr. Bowling participated in during college.
Track & Field
What is the symbol for alpha decay?
4/2 He
What is the name of group 14?
Carbon family
what is an atomic number?
The number of protons in an element
In which does the nucleus split
Fission
How many years has Mr. Bowling been teaching?
4
What decay is 0/-1 e?
Beta
Is Bromine a metal or non-metal?
Non-metal
what is the atomic number for oxygen?
8
In which a large amount of energy is released.
The icebreaker game played at the beginning of the year.
2 Truths 1 Lie
What type of decay is shown?
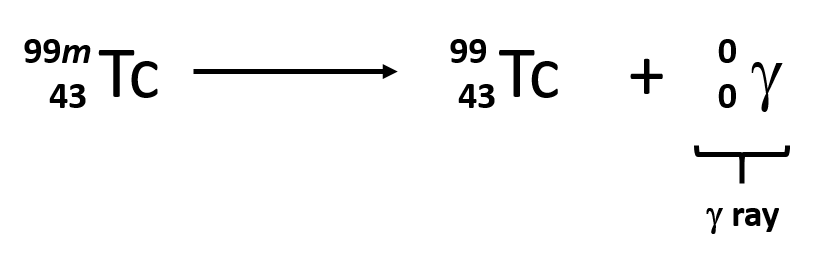
gamma
How many valence electrons does Mg have?
2
What is mass number?
The number of protons and neutrons combined.
Which has hydrogen as a reactant
fusion
The college major for Mr. Bowling (it is a type of science)
Biology
What type of decay is shown in the following reaction?
______ → +
beta
What is the least reactive group
Noble gases
What is the mass number for Ca?
40
In which are several neutrons released.
Fission
Mr. Bowling's favorite artist/musician
Halsey
What is the missing decay?
→
+ _____
alpha, 4/2 He
What is the charge of Se?
-2