What is a superscript?
a number found at the top, right of an element symbol
Ex: Mg+2 the +2 is written in superscript
How many elements in NaCl?
2
How many atoms of sodium are in 2NaCl?
2
Balanced or Not?

no
a campfire on the beach
open, because matter appears to be lost
Where are the protons located?
in the nucleus
What is the charge if there are more protons than electrons?
+, positive
What is a subscript?
a number written to bottom right of an element symbol
Ex: H2 the 2 is written in subscript
How many elements in MgSO4?
3
How many atoms of sulfur in 3MgSO4?
Balanced or not?

yes
mass stays the same before and after the reaction
closed, because matter has not been lost
Where are the electrons located?
In the electron rings (AKA atomic orbitals, energy rings, electron cloud, energy levels, etc)
What is the charge if there are more electrons than protons?
-, negative
What is a coefficient?
a number written in front of a chemical formula
Ex: 3HCl the 3 is a coefficient
How many elements in Al2(CO3)3?
3
How many atoms of oxygen are in 3 Al2(CO3)3?
27
Balanced or not?
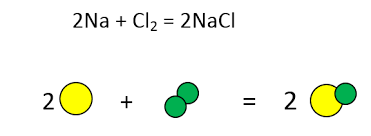
yes
gas is released into a balloon during a chemical reaction
closed, because you can catch the gas to get the same mass
Where are the neutrons located?
In the nucleus
If an atom is neutral, what 2 things are equal?
protons and electrons
what does the Law of Conservation of Matter say?
matter cannot be created or destroyed.
How many elements in NH4C2H3O2?
4
How many atoms of hydrogen are in NH4C2H3O2?
7
Balanced or not?
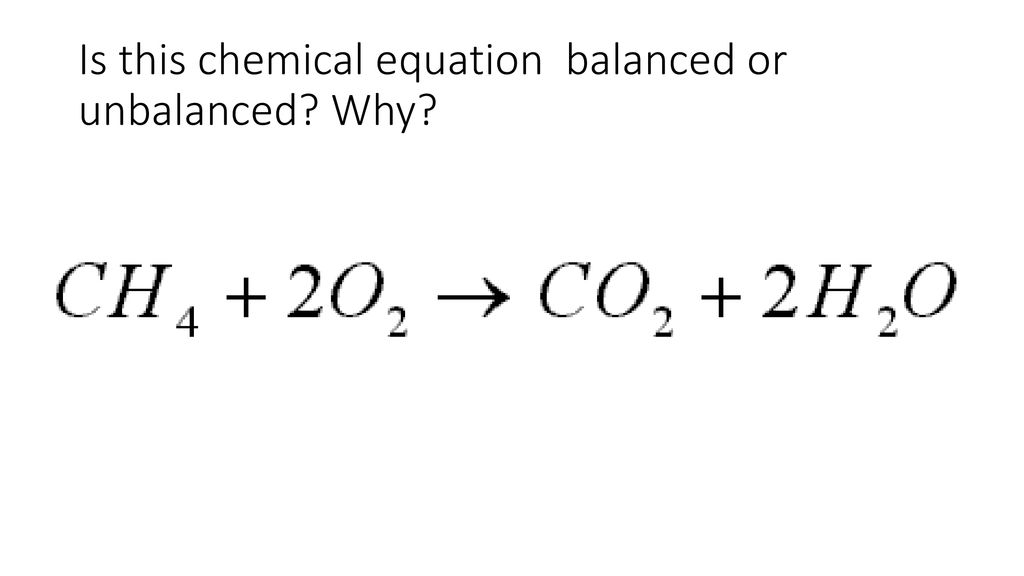
yes
2Mg + O2 --> 2MgO
closed, because the equation is balanced
Where is all of the mass of the atom located?
in the nucleus (protons + neutrons)
What particle gives an atom its identity?
protons
a system where energy can be exchanged but NOT matter.
No mass (matter) is lost & the total mass of the system remains the same.
How many elements in Ga2(HPO4)3?
4
How many atoms of hydrogen are in 2 NH4C2H3O2?
14
Balanced or not?

no
6CO2 + 6H2O → C6H12O6 + 3O2
open, because the equation is not balanced
How many electrons can fit on the 1st & 2nd energy rings?
2, 8
What are the masses of protons, neutrons, and electrons?
p = 1 AMU
n = 1 AMU
e = 0 AMU