What is a molecule?
A group of two or more atoms held together by chemical bonds
Draw the lewis structure for H2O.

Draw the lewis structure for O2.

Give one difference between an ionic and a covalent bond.
- Ionic bonds have 1 metal
- Covalent = sharing ; ionic = giving/receiving
A covalent bond is between what two types of elements?
Nonmetal + nonmetal
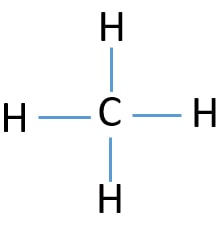
Draw the lewis structure for CH4.
Draw the lewis structure for N2H2.

Give the molecular name for HF.
Hydrogen monofluoride
An ionic bond is between what two types of elements?
Nonmetal + Metal
Draw the lewis structure for carbon tetraiodide.

Draw the lewis structure for dinitrogen.

What's wrong with the following lewis structure?
Anion - a negative ion
Cation - a position ion
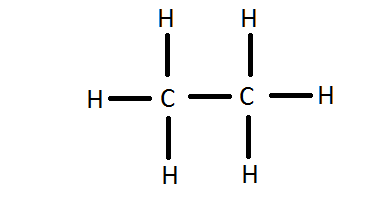
Draw the lewis structure for dicarbon hexahydride.
Draw the lewis structure for ONCl.
What is the molecular name for F2O2
Difluorine dioxide