CO2
Carbon Dioxide
Bromine Monofluorine
BF
What types of atoms form a covalent bond
nonmetals only
Draw the correct dot formula for HCl

Dihydrogen Monoxide
H2O (water)
NH3
Nitrogen Trihydride
Nitrogen Dioxide
NO2
Can covalent bonds conduct electricity as a solid, in solution, both or neither?
Neither
Draw the correct dot formula for CH4

FeO
iron(II) oxide
PO5
Phosphorous Pentoxide
Carbon Tetrahydride
CH4
Covalent bonds typically have very high or low melting points?
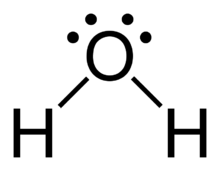
Draw the correct dot formula for H2O
silver bromide
AgBr
S2F6
Disulfure Hexafluoride
Tribromine Octoxide
B3O8
In covalent bonds, the electrons are
shared
Draw the correct dot formula for CO2

MgF2
magnesium fluoride
N2O7
Dinitrogen Heptoxide
P5F7
If the electrons are being shared UNEQUALLY, this is a
polar covalent bond
Draw the correct dot formula for CH3OH

C4H6
tetracarbon hexahydride