True or False: Ionic bonds are made of only nonmetals.
What is false?
True or False: In covalent bonding, each atom needs its own eight electrons.
What is false?
KBr (potassium bromide)
Ionic
CCl4
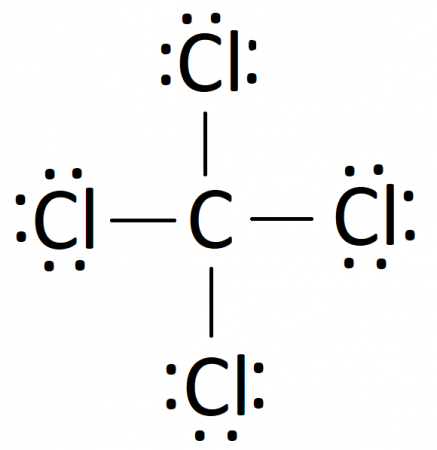
True or False: Covalent compounds share electrons.
What is true?
How many lone pairs does nitrogen have in this lewis dot structure?

1
O2
Covalent
NH3

The transfer of electrons from one atom to another
What is ionic?
How many shared pairs of electrons does nitrogen have?

3
CO2
Covalent
H2CO

A molecule that shares electrons equally
What is nonpolar covalent?
Provide the Lewis dot structure of hydoxide (OH-).
Copper chloride (CuCl2)
Ionic
NH4+
The unequal sharing of electrons in a molecule
What is polar covalent?
The valence electrons that are not shared with another atom.
lone pair
H2O2
Covalent
C2H3N
