In a covalent bond valence electrons are ________.
Shared
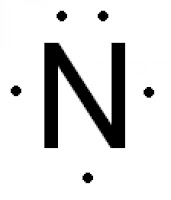
What is the lewis dot structure for a N atom?
Name one molecular structure.
Linear, trigonal planar, tetrahedral, pyramidal, bent
Nonpolar
What does a dot represent in a lewis dot structure?
An electron
How many valence electrons does hydrogen have?
1
The lines between atoms in a lewis dot structure represent what subatomic particle and how many are indicated by a single line?
2 electrons
What is the angle in a linear molecule?
180 degrees
Negative
Does a double bond off the central atom of a molecule constitute 1 or 2 electron domains?
One
How many valence electrons does Carbon have?
4
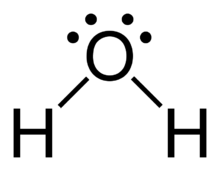
What is the lewis dot structure for water?
What is the molecular structure for a molecule with 3 electron domains?
Trigonal planar
Is water polar or non-polar?
Polar
Which of the three 4 domain molecular structures has the largest bond angles?
tetrahedral
How many valence electrons does Flourine need to fill it's outer shell?
1
How many unshared electron pairs (2 dots is a pair) are there in a lewis dot structure for cabon dioxide?
4
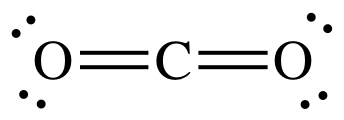
What is the molecular structure of a water molecule?
Bent
Is methane (CH4) polar or nonpolar?
Nonpolar
What is the lewis dot structure for NH3?

What type of element has a full valence outer shell?
Nobel gases
What is the lewis dot structure for cyanide (HCN)?

What is the molecular structure of methane (CH4)?
Tetrahedral
What is the polarity of carbon dioxide?
What is the lewis dot structure, molecular geometry, and polarity for CHClO?
Trigonal planar, polar