This is what happens to electrons in a covalent bond
What is shared
The relative boiling point of molecular compounds
What is low
When drawing the Lewis structure of an ion this is necessary
What is brackets and the charge?
the meaning of the acronym VSEPR
What is Valence Shell Electron Pair Repulsion
Bond polarity measures the differences in this
What is electronegativity
formed when two pairs of electrons are shared in covalent bond
What is double covalent bond
The type of bond in a molecule
What is covalent
These are the names of the trio in Harry Potter that bond after fighting a troll.
Who are Harry, Ron, & Hermione?
The shape of H2O
What is bent?
A polar molecule contains a permanent this
What is a dipole
A pair of electrons that are not bonded
What is an unshared pair
Shows how many atoms of each element a molecule contains
What is molecular formula
A structure that occurs when it is possible to draw two or more valid Lewis structures
What is resonance structure
A central atom with three bonded atoms and one lone electron pair
What is trigonal pyramidal?
The symmetry of ethene (C2H4) makes it this
What is nonpolar
Nitrogen forms one of these with itself in N2
What is a triple bond
O2 is this type of molecule
What is diatomic
The shape of SO2
What is bent? resonance!!
resonance!!
The shape of CH4
What is a tetrahedral?
This type of dipole-dipole IMF is formed when a hydrogen atom is bonded with Nitrogen, oxygen, and fluorine.
What is hydrogen bonds
The lewis structure of CO2
What is ::O=C=O::
The Lewis structure for NH3
What is 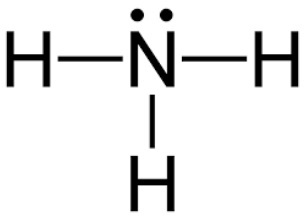
The reason SF6 is special
What is it has an expanded valence?
The shape for each carbon in C2O42-
What is trigonal planar?
This is the weakest IMF
What is London dispersion force