How many atoms in a molecule of Methane; CH4?
5
1 carbon atom
4 hydrogen atoms
Which state of matter (solid, liquid, or gas) has a definite shape and a definite volume?
Solid
Explain how you know
Physical
Write the chemical formula of the missing product 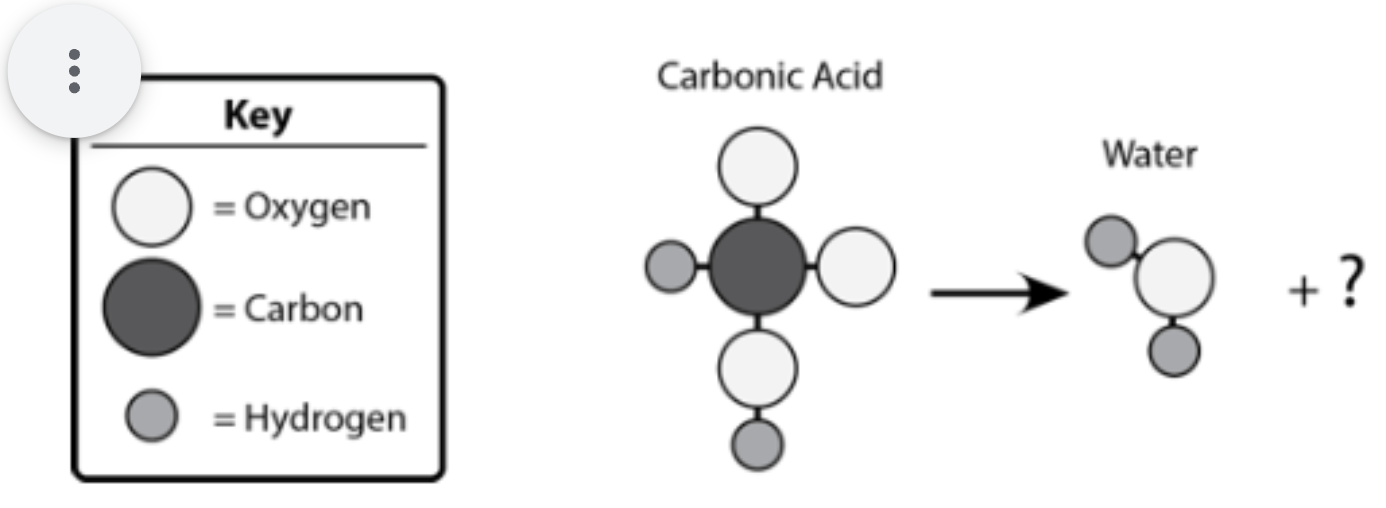
CO2
a ______ material is one that can be found on earth without human intervention.
natural
How many elements are found in one molecule of CO2 ?
2, Carbon (C) and Oxygen (O)
Which state of matter has the MOST thermal energy ?
gas
17. Which of the following are REACTANTS of this chemical reactiON?
CO₂ + H₂O → C₆H₁₂O₆ + O₂.
CO₂ + H₂O
When the number of the reactant's atoms and the number of each product's atoms are equal, the chemical reaction is______________
Balanced
Give an example of a synthetic material
answers may vary
Diamond and graphite are two different substances, yet both are made up of pure carbon atoms (C)
How is it possible that they have the same formula, but are all different substances?
The way the carbon atoms are arranged makes them different.
The carbon atoms are arranged in different patterns.
What happens to the thermal energy of liquid water molecules if it is turned into ice?
They slow down because it is going from a liquid to a solid.
What are the five signs of a chemical reaction?
Bubbles/gas, light, precipitate forms, change in temp, change in color
The _______________________ is the number written below and to the right of a chemical symbol to show how many atoms are present.
subscript
How is a synthetic material related to a natural resource?
Synthetic materials are made from natural resources
Draw a molecule of H2O
drawing should show a molecule with 2 hydrogen atoms and 1 oxygen atom
The starting temperature of two rectants was 12 degrees. At the end of the reaction, the temperature measured was 20 degrees.
Is this an example of an endothermic or exothermic reaction? How do you know?
the temperature increased, the reaction must have released heat — making it exothermic.
What happens to the atoms of the reactants during a chemical reactions?
They are rearranged to form a new substance (products)
Why does this picture demonstrate the law of conservation of mass? 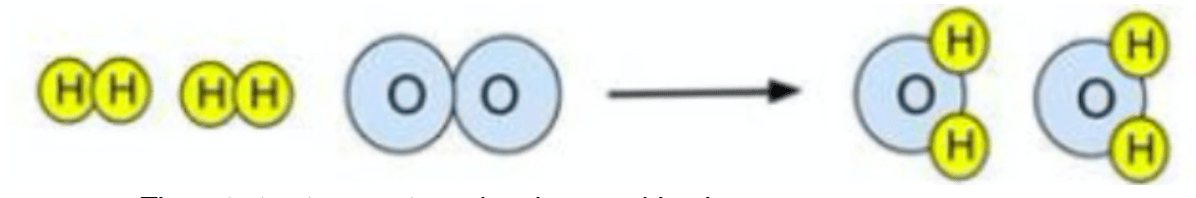
The number of atoms of each element are the same on both sides
What is Kevlar?
a synthetic material, used for bullet proof vests.
The formula of glucose is C₆H₁₂O₆. How many atoms does 1 molecule of glucose have?
24
What would happen if room temp water is set on hot stovetop? The temperature of the water begins to _____________and the thermal energy of the water_______________.
increase, increase
Give one example of a physical change. Give one example of a chemical change.
answers may vary
If reactants had a mass of 20g when he started, and the product had a mass of 18g. Explain what happened.
The mass of the gas that was produced was not able to be measured since the container was open.
Name two fabrics that are NATURAL materials. name two fabrics that are SYNTHETIC materials
Natural: cotton, silk, wool
Synthetic: Nylon, Polyester, rayon, spandex