Is Gold (Au) an element or a compound?
Element
How many total atoms are in the chemical formula H2O?
3
Shredding paper is an example of a....
Physical Change
What is the most common solvent in aqueous solutions?
Water
How does increasing temperature affect the rate of dissolution?
increases the rate of dissolution /
causes the solute to dissolve faster
What do you call a molecule made of two oxygen atoms?
O₂
How many oxygen atoms are in CO₂
2 oxygen atoms
What type of change is rusting iron?
chemical change
The two components of a aqueous solution.
What are solute and solvent
What is the rate of dissolution?
How quickly a solute dissolves into a solvent.
What is the smallest unit of an element?
An Atom
How many atoms are in the formula C₆H₁₂O₆?
24 atoms
Bubbles forming and releasing gas as a powder is added to a clear liquid is an example of a....
Chemical Change
If the dilution of a solution is decreasing, then the concentration of the solution is _________.
Increasing
What effect does stirring have on the rate of dissolution?
increases the rate of dissolution/
causes the solute to dissolve faster
Which substances are compounds?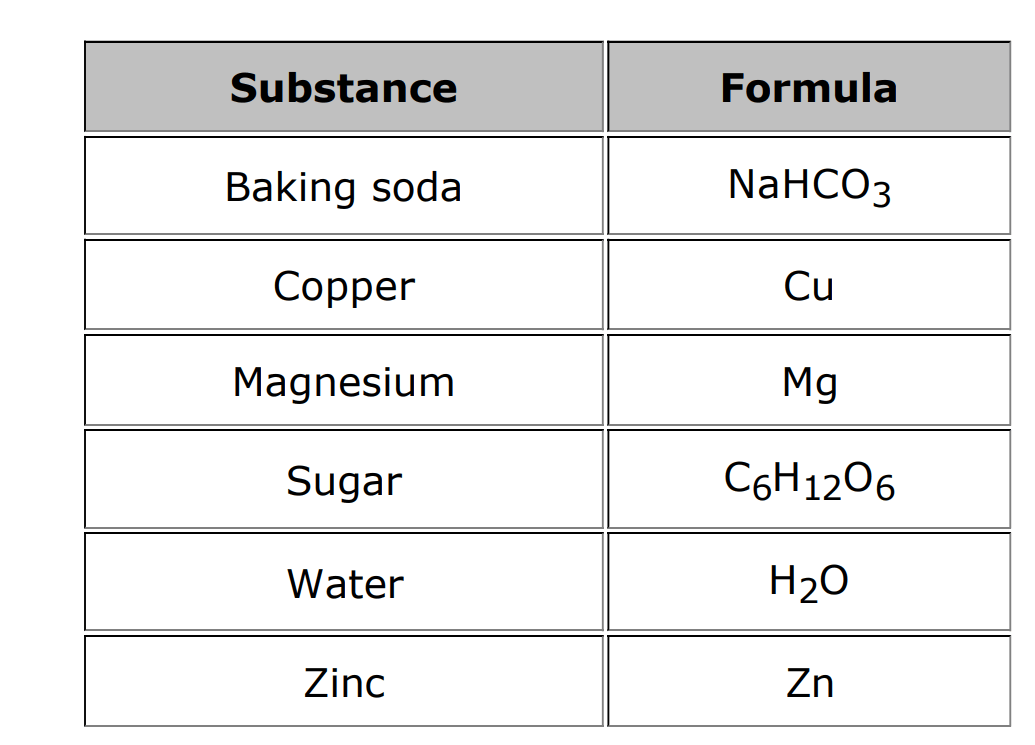
Baking soda, sugar, water
This table shows the number of atoms in Sodium Sulfate. Using your Periodic Table and the table below, write the correct chemical formula for Sodium Sulfate using this table.
Hint: (sodium)amount (sulfur)amount (oxygen)amount 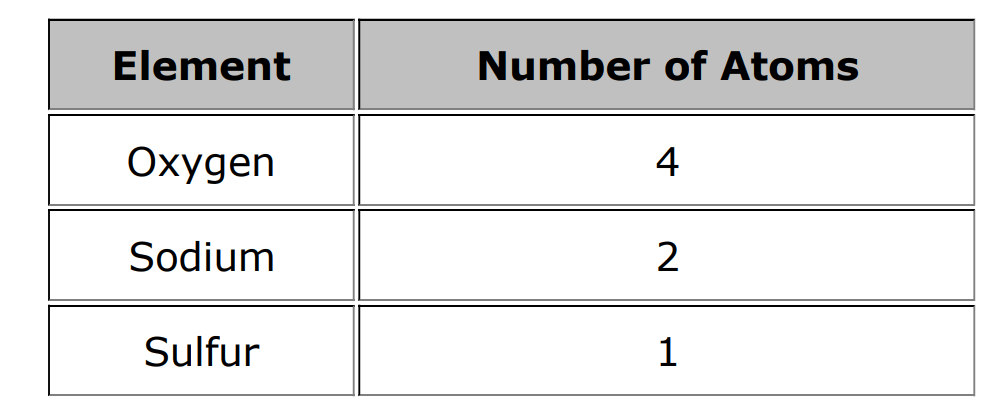
Na2SO4
A state of matter change (ex. liquid to gas) is an example of a....
Physical Change
What happens to the concentration when you add more solvent?
it becomes more dilute
How does increasing the surface area (crushing) of a solute affect its dissolution rate?
increases the rate of dissolution /
causes the solute to dissolve faster
Which of the following best describes the model pictured below? Explain why on the whiteboard.
A. It is an element found on the Periodic Table.
B. It is an element because it has only one type of atom.
C. It is a compound because it has only one type of atom.
D. It is a compound because it has different types of atoms.
D, because the model shows different types of atoms.
How many total atoms are present in this chemical formula: 3C2(H4O8)2
Total Atoms: 78
Write as many clues of a of a chemical change you can remember from class in 40 seconds.
Light Produced
Temperature Change (i.e. gets hot / gets cold)
Smell Produced
Gas Produced / Fizzing / Bubbling
Unexpected Color Change
Food Spoiling
Smoke
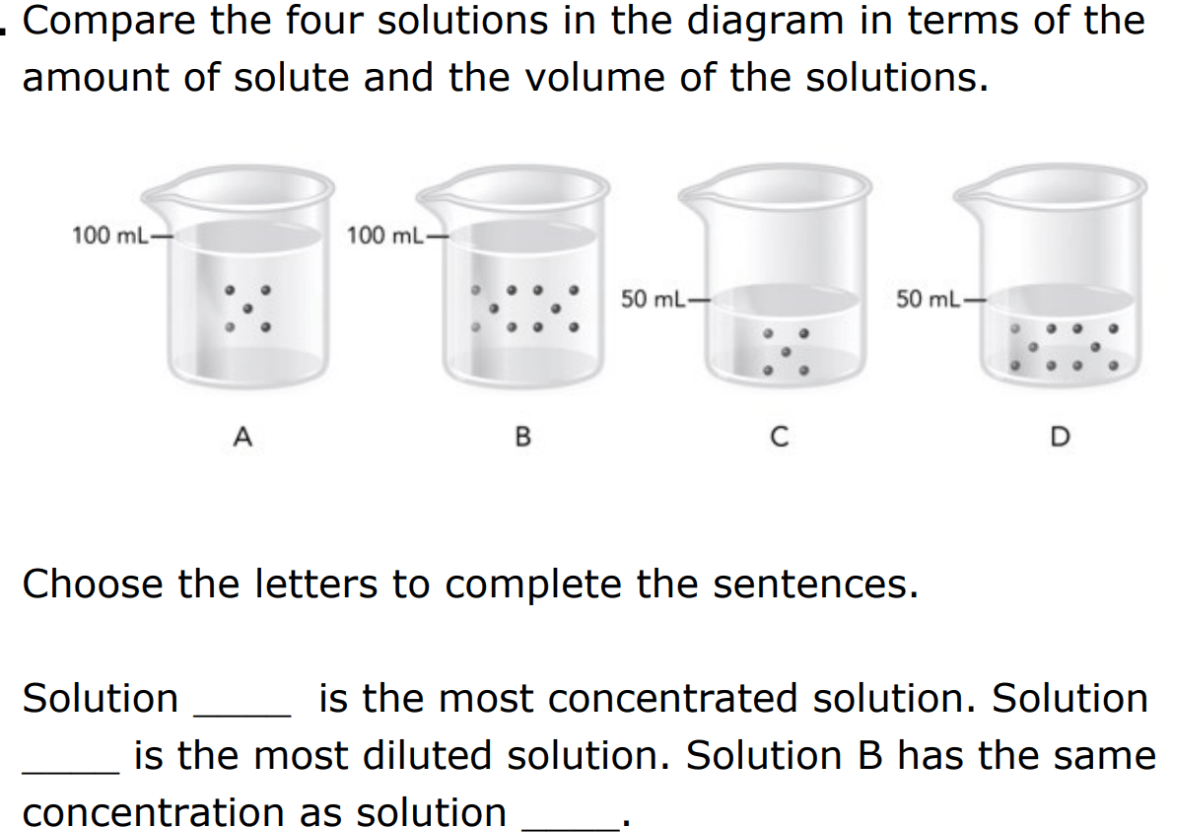 Place letters A, C, and D in the correct order to fill in the blanks.
Place letters A, C, and D in the correct order to fill in the blanks.
D, A, C
The 3 factors that affect rate of dissolution.
What are: Temperature, surface area, agitation