(Using Thermal Energy and it's sources)
(Using Thermal Energy and it's sources)
(Measuring Temperature)
(The Particle Model of Matter, Temperature, Thermal Energy, and Changes of State)
(Expansion & Contraction)
(Transferring Energy + Conserving our Fossil Fuels)
The energy generated by the movement or vibration of particles; the total kinetic energy of all the particles in a substance. ("Heat Energy")
What is thermal energy?
A relative measure of how hot or cold something is, measured on a scale; the average kinetic energy of the particles in a substance
What is temperature?
Of a material, the energy change that is required to warm or cool a standard amount of the material (1 g or 1 kg) by 1°C
What is specific heat capacity?
The different forms (solid, liquid, or gas) that matter can take.
What are the (3) States/Phases of Matter?
Radiation, Conduction, and Convection.
What are the 3 ways energy can be transferred?
Since the beginning of time, uncontrolled heat has scorched and spoiled the taste of food and has destroyed buildings and homes.
What are some of the problems people have experienced by using energy from heat?
(Topic 1, pg. 188)
A device used to measure temperature. Your senses can be easily fooled, but this device is more reliable. This device can be either mechanical or electrical.
*Hint: Galileo invented one of the first ones around 1600. See image below.

What is a thermometer?
• All substances are made of tiny particles too small to be seen.
• The particles are always in motion — vibrating, rotating, and (in liquids and gases) moving from place to place.
• The particles have spaces between them.
• The motion of the particles increases when the temperature increases. The motion of the particles decreases when the temperature decreases.
What are the 4 points of the Particle Model of Matter?
 • Of substances, to increase in volume, usually when warmed.
• Of substances, to increase in volume, usually when warmed.
• Of substances, to decrease in volume, usually when cooled.
1. What is Expansion?
2. What is Contraction?
When a fire burns without enough oxygen, a colorless, odorless, gas is produced.
What is carbon monoxide + How is carbon monoxide produced?


• A type of heating that uses materials in a structure to absorb, store, and release solar energy.
• A type of heating that uses mechanical devices like fans to distribute stored thermal energy.
• Passive Solar Heating
• Active Solar Heating

-273.15 °C or 0 on the ___________ Scale, which is mainly used for scientific purposes, is known as ___________.
*Hint: The second blank refers to the coldest possible temperature ever.
• Kelvin
• Absolute zero

"Proper" names for all!
• To turn from a solid to a liquid
• To turn from a liquid to a solid
• To turn from a liquid to a gas
• To turn from a gas to a liquid
• To turn from a solid to a gas
• To turn from a gas to a solid
• What is melting (fusion)?
• What is freezing (solidification)?
• What is evaporation?
• What is condensation?
• What is sublimation?
• What is sublimation/deposition?
________ Have a definite shape and volume and can't be compressed.
________ Have a definite volume but no definite shape. (Takes shape of container). Almost incompressible.
________ Have no definite shape or volume. (Takes shape of container). Easily compressible.
• Solid
• Liquid
• Gas
Energy Source, Direction of Energy Transfer, Transformations, Waste Heat, and Control Systems.
What are the 5 features of all energy transfer systems?

This is...
Another problem occurs when fossil fuels burn and produce carbon dioxide gases. Carbon dioxide is required by green plants, as you saw in Unit 1. It occurs naturally in the atmosphere. You add carbon dioxide to the air every time you breathe out, because as your body uses food, it produces carbon dioxide as a waste product. With ever-increasing numbers of planes, trucks, and automobiles meeting the demands of industry, natural carbon dioxide recycling systems are becoming badly overloaded. What happens then? Heat from Earth is unable to escape into space because it is trapped by greenhouse gases (largely carbon dioxide) in the atmosphere.
What is the greenhouse effect?
What do all these temperatures signify?
(Answer all)
• 37 °C
• 0 °C
• 20 °C
• 100 °C
• -273.15 °C
• What is normal human body temperature?
• What is the freezing/melting point of pure water at sea level?
• What is the average normal room temperature?
• What is the boiling point of pure water at sea level?
• What is "absolute zero"?
![]()
A process in which the faster-moving particles on the surface of a liquid evaporate and escape into the air; the slower-moving particles, which are left behind, have lower kinetic energy, decreasing the temperature of the remaining liquid and the surface on which it is resting.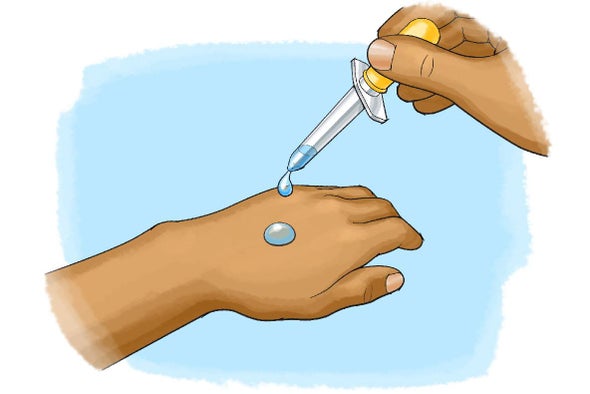
What is evaporative cooling?
What season is it in the first picture? What about the second one?
1.

2.

1. Summer (Expansion/close together)
2. Winter (Contraction/far apart)
A burn caused by touching a hot metal pot is painful. The effects of a forest fire are devastating. Thermal energy does indeed have the power to hurt and destroy us, our belongings, and our environment. Storage of fossil fuels presents a fire hazard, but there are other risks, as well.
What are some of the dangers involved when thermal energy is used?
Directly from the homework:
Hammering a nail into a piece of wood and the wood heats up. Compressing air in a bicycle pump, and the valve on the pump becomes warm.

What are 2 ways of converting mechanical energy to thermal energy?
(Topic 7, Pg. 240)

• In a ______________, wires made of two different metals are twisted together. When the twisted wire tips are heated, a small electrical current is generated. The amount of current depends on the temperature of the wires. The electrical current from the device can be used to turn a switch or a valve on or off if the temperature changes.
• A ____________ is made of two different metals joined firmly together. As the strip is heated, one metal expands more than the other. The strip is forced to coil more tightly. When the strip cools, the process is reversed. The same metal that expanded rapidly now contracts rapidly and the strip uncoils again. Movements of the strip can operate a type of electrical switch, which can be used to control furnaces, air conditioners, refrigerators, or other devices.
• In one type of ____________, a bimetallic strip coils and uncoils as the temperature changes. One end of the strip is attached to a long, light metal lever that holds a special pen. Tiny movements of the bimetallic strip cause much larger movements of the free end of the lever and the pen. The pen traces a rising and falling line on a strip of paper attached to a slowly turning drum. The drum usually makes one turn every seven days, so each strip of paper contains a record of temperature changes for an entire week.
• This: 
• What is a thermocouple?
• What is a bimetallic strip?
• What is a Recording Thermometer?
• What is an Infrared Thermogram?
The smallest particles of matter are too tiny to observe clearly. They can be observed only in large groups. In any substance, some particles always seem to be moving faster than average. Other particles seem to be moving unusually slowly. The average speed of many particles, however, is always indicated by their temperature.
Why is it so hard to test the particle model to see if it is correct?
Directly from the homework:
Which state of matter shows the largest change in volume when warmed or cooled? Which state shows the smallest change?
Gas shows largest change, therefore solid shows smallest change.
(Pg. 214)
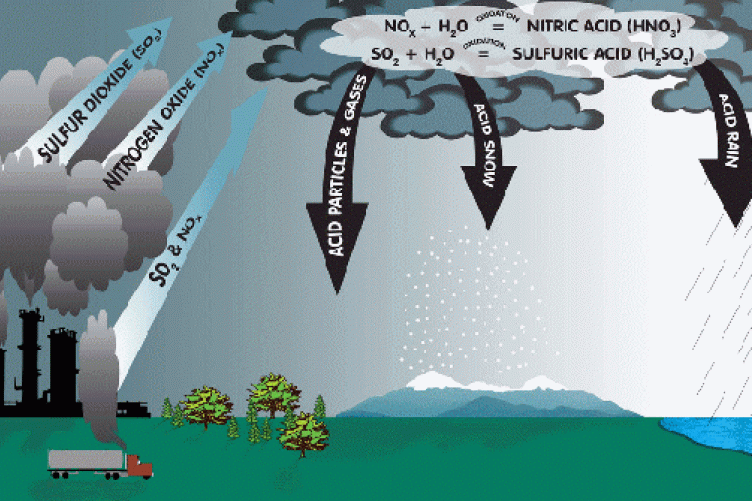
A gas that is extremely irritating to the eyes, nose, and throat. People with asthma suffer greatly from this pollutant. Hydro-electric companies invest large amounts of money into researching and developing technology that will remove or reduce emissions of it.
*Hint Hint Excessive amount in atmosphere leads to acid rain
Gif of the gas's atoms:
What is sulfur dioxide (g)?