Which of the following elements would contain the valence electron configuration 4s24p5?
A. Fluorine
B. Sulfur
C. Calcium
D. Bromine
E. Antimony
D. Bromine
Which one of the following reactions of alkenes does the Anti-Markovnikov’s rule apply instead of Markovnikov’s rule?
A. Hydration
B. Oxymercuration-demercuration
C. Hydroboration-oxidation
D. Addition of HCl
E. Addition of Br2
C. Hydroboration-oxidation
In an emergency, an individual with type AB antigen in their red blood cells
A. may receive a transfusion of type O blood.
B. may receive a transfusion of type A blood.
C. may receive a transfusion of type B blood.
D. All of the above
E. None of the above
D. All of the above
How many cubic centimeters of water will approximately be required to fill a cylinder with a 3 cm radius and 7 cm height?
A. 63
B. 66
C. 132
D. 198
E. 496
D. 198
In a reaction between Mg and Ar, which of the following is most likely to occur?
A. no reaction would occur
B. a precipitate would form
C. A gas would be produced
D. a color change would occur
E. an aqueous solution would form
A. no reaction would occur
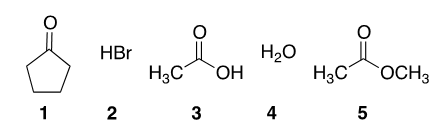
Place the following in order of LEAST acidic to MOST acidic.
A. 1 < 5 < 3 < 4 < 2
B. 5 < 1 < 3 < 2 < 4
C. 1 < 5 < 4 < 3 < 2
D. 5 < 1 < 4 < 3 < 2
E. 2 < 3 < 4 < 1 < 5
D. 5 < 1 < 4 < 3 < 2
C6H12O6 + O2 CO2 + H2O
This process is completed...
A. in the cytoplasm.
B. in the area of the cell membrane.
C. in the nucleus.
D. in the mitochondria.
E. in the area around the ribosomes.
D. in the mitochondria.
There are 5 white, 7 red and 8 black balls in a box. If you are blindly picking up balls from the box, what is the minimum number of balls you need to take out of the box to ensure that you have at least 2 balls of the same color?
A. 2
B. 3
C. 4
D. 5
E. 6
C. 4
Which one of the following reactions will be accompanied by an increase in entropy?
A. Na(s) + H₂O(l) → NaOH(aq) + H₂(g)
B. I₂(g) I₂(s)
C. H₂SO₄(aq) + Ba(OH)₂(aq) → BaSO₄(s) + H₂O(l)
D. H₂(g) + 1/2 O₂ (g) → H₂O(l)
E. None of the above.
A. Na(s) + H₂O(l) → NaOH(aq) + H₂(g)
Which diene would be more reactive in a Diels-Alder reaction and why?
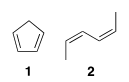
A. 2, because it can transform into the cis conformation
B. 2, because it is in a trans conformation
C. 1, because it is very electrophilic
D. 1, because it can transform into a trans conformation
E. 1, because it is in a cis conformation
E. 1, because it is in a cis conformation
Which of the following illustrates the principle of induction in invertebrates?
A. In an embryo, the presence of a notochord beneath the ectoderm results in the formation of a neural tube.
B. A neuron synapses with another neuron via a neurotransmitter.
C. Eye muscles constrict in response to light.
D. Secretion of TSH stimulates the secretion of thyroxine.
E. The maternal parent induces spontaneous expression of recessive genes.
A. In an embryo, the presence of a notochord beneath the ectoderm results in the formation of a neural tube.
Mark’s car began a journey from a point at 7 AM, traveling at 30 mph. At 8 AM Jason’s car started traveling from the same point at 40 mph in the same direction as Mark. At what time will Jason pass Mark?
A. 11 AM
B. 10 AM
C. 10:30 AM
D. 11:30 AM
E. Jason never passes Mark
A. 11 AM
For a mixture of gases in a container, the total pressure is equal to __________ because of what law?
A. The pressure of the largest gas, First Law of Thermodynamics
B. The pressure of the smallest gas, First Law of thermodynamics
C. The sum of the partial pressures of all gases, Dalton’s law of partial pressure
D. The pressure of the greatest gas, Dalton’s law
E. The sum of the top two gases in the mixture, Dalton’s law of partial pressure
C. The sum of the partial pressures of all gases, Dalton’s law of partial pressure
Which of the following statements best describes the structural relationship between cis-1,2-dibromocyclopropane and trans-1,2-dibromocyclopropane?
A. The two compounds are mirror images of one another
B. The two compounds can be separated by ordinary physical-chemical separation methods
C. The two compounds have the same melting points
D. All of the above
B. The two compounds can be separated by ordinary physical-chemical separation methods
Cells that are involved in active transport, such as cells of the intestinal epithelium, utilize large quantities of ATP. In such cells, there are
A. high levels of adenylate cyclase activity.
B. many polyribosomes.
C. many mitochondria.
D. high levels of DNA synthesis.
E. many lysosomes.
C. many mitochondria
n a certain course, the Professor has two different grading policies, from which he picks the best. The first policy is to put 40% and 60% weights, respectively, on the midterm and final exams. Under the second grading policy, these weights are 50% each. A student has scored 85% on the midterm, and needs to score at least x% on the final to make a B (a total of 82%). What is the value of x?
A. 82
B. 80
n a certain course, the Professor has two different grading policies, from which he picks the best. The first policy is to put 40% and 60% weights, respectively, on the midterm and final exams. Under the second grading policy, these weights are 50% each. A student has scored 85% on the midterm, and needs to score at least x% on the final to make a B (a total of 82%). What is the value of x?
A. 82
B. 80
C. 79
D. 81
E. 78
C. 79
The hardest substance known is diamond. Which best describes the structure of a diamond?
A. A 3D network lattice of covalently bonded carbon atoms.
B. A 2D network lattice of covalently bonded carbon atoms.
C. A 3D network lattice of carbon ions held together by ionic bonds.
D. A 3D network lattice of carbon ions in a sea of mobile valence electrons held together by metallic bonds.
E. A 3D network lattice of carbon atoms held together by hydrogen bonding and dispersion forces.
A. A 3D network lattice of covalently bonded carbon atoms.
The 1H NMR spectrum of an unknown compound shows the following signals: triplet, quintet, and triplet (relative integrals 1:2:3, respectively). Which compound is consistent with this data?
A. 1,1-dibromopropane
B. 1,2-dibromopropane
C. 1,3-dibromopropane
D. 2-bromopropane
E. 1,1-dibromoethane
A. 1,1-dibromopropane
In the process of glycolysis...
A. NAD+ is reduced to NADH
B. The final product is acetyl coenzyme A
C. Oxygen is required for maximal ATP production
D. There is a net production of 4 ATP per glucose molecule
E. Fructose-1,6-bisphosphate is converted into acetyl coenzyme A
A. NAD+ is reduced to NADH
X is a multiple of A. A is always even, and is always divisible by B. X is also never divisible by C. Which of the following statements must be true?
A. A is divisible by C.
B. X is divisible by B.
C. Division of X by A equals B.
D. B is divisible by C.
E. A * X is always odd.
B. X is divisible by B.