Which jar has a higher density?
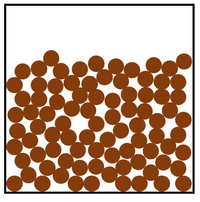
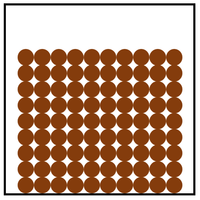
What is, the bottom jar has a higher density because the particles are more closely packed together?
Liquids are ____ dense than gases and _____ dense than solids.
What is more dense than gases and less dense than solids?
The formula for calculating density is _____.
d=mass divided by volume
Do objects with a lower density float or sink when paired with something of a higher density?
What is, lower density will float?
Does water vapour sink or float?
What is float? It will sit on top of fluid and escape a jar if possible.
The units g/mL and g/cm3 represent the _________ of a substance.
What is density?
True or false: the shape of an object impacts the density
What is true?
Which picture shows a liquid?
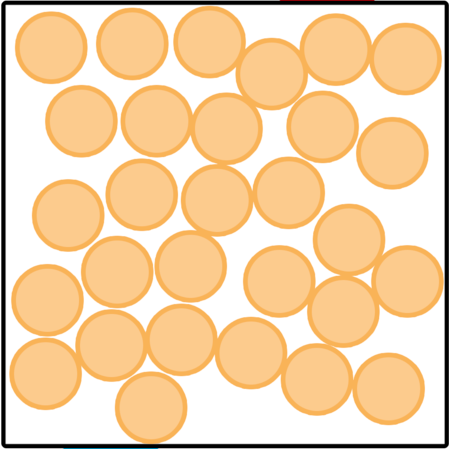
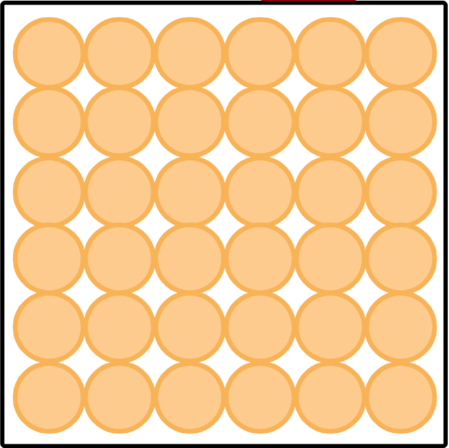
What is, the first picture shows a liquid?
When we see the unit cm3
we know that it is the volume of a __________.
What is a solid?
True or false: density is influenced by how closely particles are together
What is true? Closer = more dense, further apart = less dense
Why do solids usually sink in water?
What is, because it has a higher density? It has more particles and they are more closely packed together.
Sometimes, the liquid state of a substance can have a ____ density than the solid state.
What is higher density?
Density = the amount of ______ in a certain volume.
What is mass?
Gases have _____ space between particles.
What is less space between particles?
Even though wood and ice are solids (and should be more dense than liquids), they float on water. Why?