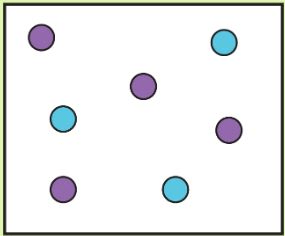
Tell me which is high density and which is low density
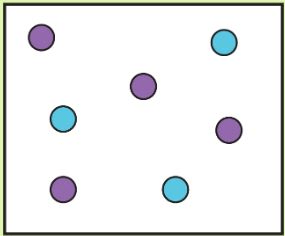
High Density:
Low Density:
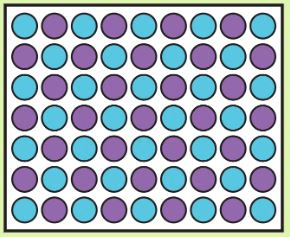
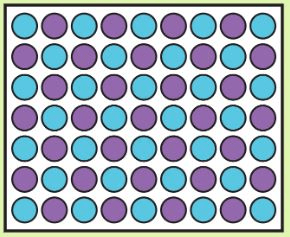
box a has ______ mass than box b
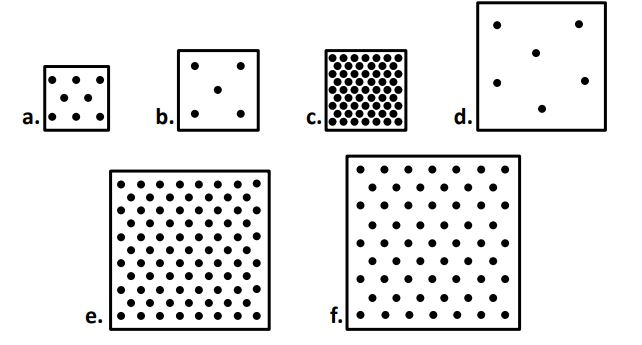
more/greater/larger/higher
Water has a density of 1 g/mL, estimate the density of the following:
Ice, which floats on water.
A rock which sinks in water.
Ice: < 1 g/mL (less than)
A rock: >1 g/mL (greater than)
Calculate the density of an object if the given volume is 20 cm and the mass is 80 g.
D = M/V
D = 80/20
D = 4 g/cm3
Calculate the density of an object if the given volume is 40 cm and the mass is 80 g.
D = M/V
D = 80/40
D = 2 g/cm3
Units for mass
grams (g)
kilograms (kg)
The amount of space something takes up
volume
box ____ and ____ have the same volume
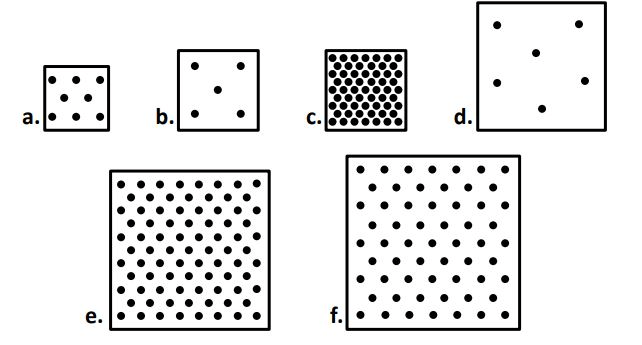
B & C
between boxes C, E, & F, box ____ has the largest volume
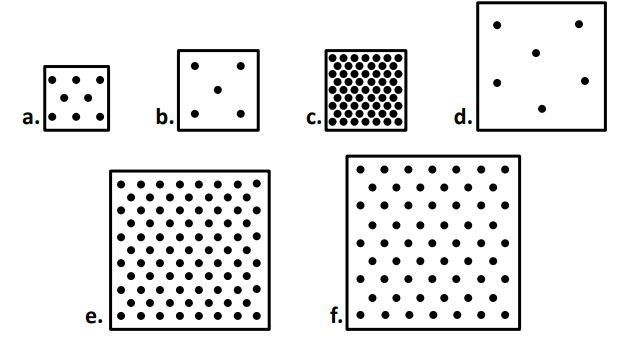
F
Ball A sinks in a beaker full of a mysterious liquid. That liquid has a known density of 2 g/mL

> 2 g/mL
Calculate the density.

Volume of a regular solid = L x W x H
4 x 4 x 4 = 64
D = M/V
D = 640 / 64
D = 10 g/cm3
Jamal wants to calculate the density of a figurine he found in his collection. He puts it on a scale and gets a value of 99 g. When he drops it into a graduated cylinder, the water level raises 11 mL. What is the density of the figurine?
Volume of irregular objects = water displacement
V = water ending level - water starting level
V = 11 mL
D = M/V
D = 99/11
D = 9 g/cm3
units for volume of a liquid
milliliters (mL)
the amount of matter in an object
mass
when comparing boxes A - D, box ____ has the largest mass but box ___ has the smallest.
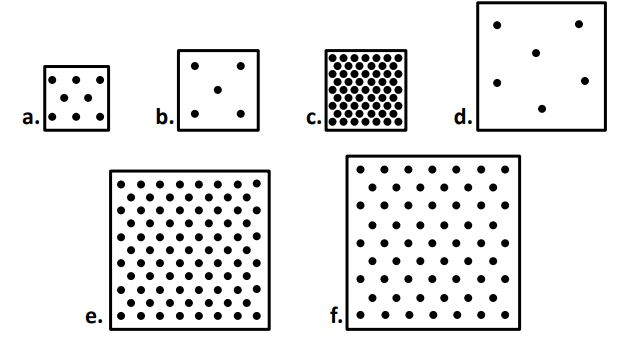
C
B
when comparing boxes A - D, box ____ has the largest volume but box ___ has the smallest.
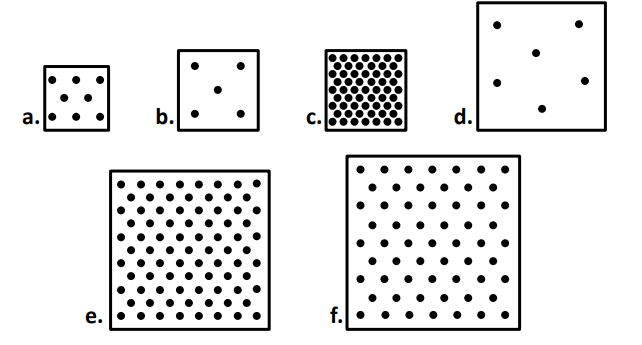
D
A
Both of these objects float in this mystery liquid. The density of the liquid is known to be 3g/mL. BOTH objects have an estimated density of

< 3 g/mL
Calculate the density of an object who has side lengths of 1 cm, 2 cm, and 4 cm and a mass of 80 g.
Volume of a regular solid = L x W x H
V = 1 x 2 x 4
V = 8
D = M/V
D = 80/8
D= 10 g/cm3
Walter measures 55 mL of water into a graduated cylinder. He measures the mass of a rock to be 189 g. When he drops the rock into the graduated cylinder, the water level raised up to 73 mL. He measured the height of the rock to be 13 cm. What is the density of the rock?
Volume = water displacement method
V = Ending water level - starting water level
V = 73 mL - 55 mL
V = 18 cm3 (because mL and cm3 are equivalent but mLs are used for liquids and the rock is a solid)
D = M/V
D = 189/18
D = 10.5 g/cm3
units for volume of a solid
cubic centimeters (cm3)
the amount of matter packed into a certain amount of space
density
When comparing ALL the boxes, the box with the highest density is. Which is the lowest density?
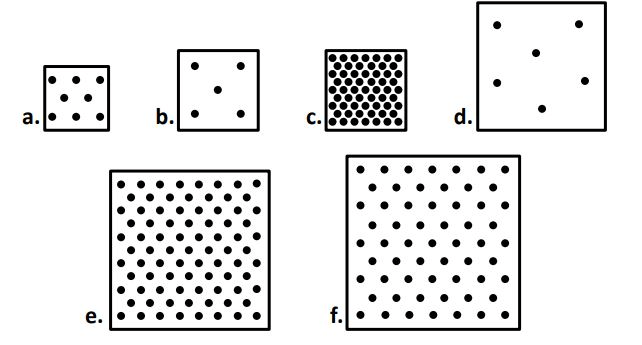
C
D
When comparing ALL the boxes the ONE of the sets of boxes with the most similar densities are... (there are two possible sets, I only need one)
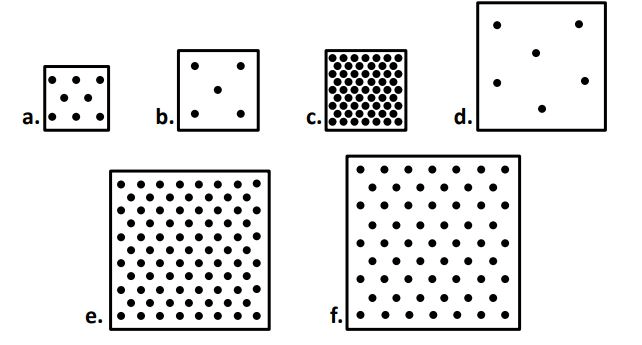
A & F
E & F
No matter where you place this ball, it stays suspended in this liquid. The density of this liquid is 4 g/mL.

The ball has a density approximately equal to the density of the liquid because it stays suspended in the middle/wherever you place it. So approximately equal to 4 g/mL.
Calculate density

V = L x W x H
V = 7 x 1.5 x 7
V = 73.5 cm3
D = M/V
D = 88.2/73.5
D = 1.2 g/cm3
If this mineral has a mass of 900g, what is its density?
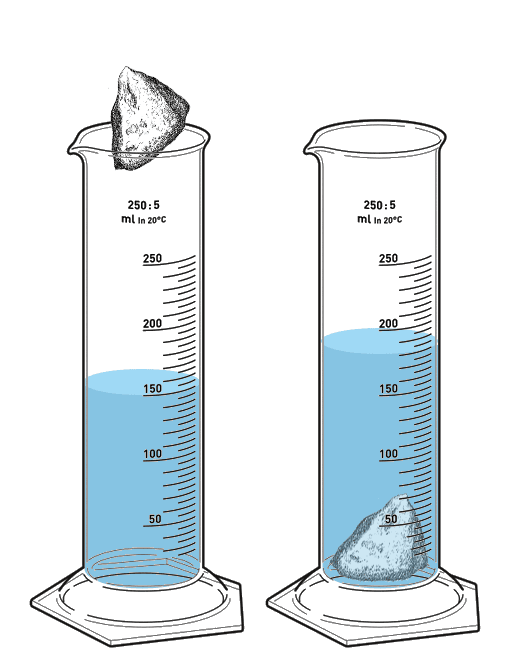
Volume of irregular object = water displacement
V = ending water level - starting water level
Big lines are marked at 50s so small lines have to be 5's (If you start at 100 and count by 5's, you get to 150. You don't get to 150 if you count by anything else)
V = 180 - 150
V = 30 cm3 (because mL and cm3 are equivalent but mLs are for liquids and the rock is a solid)
D = M/V
D = 900/30
D = 30 g/cm3
Units for density (give answers for both solids and liquids)
grams per milliliter (g/mL)
grams per cubic centimeter (g/cm3)
Identify whether each of the statements below are true or false. If they are false, correct them to make them true.
1. Cutting a piece of bread in half means the density will be half what it was when it was a full piece of bread because you have half the amount of bread.
2. Cutting a piece of bread in fourths means the density will be one-fourth what it was when it was a full piece of bread because you have one-fourth the amount of bread.
3. Taking 2 pieces of bread instead of 1 piece will cause the density to be double because you have double the amount of bread.
All of these are false. Changing the amount of a substance does not change the density of the substance. If the density of the bread was 2, then the density of the 1/2 piece is 2. Then density of the 1/4 piece is 2. The density of 2 pieces is 2. Density is a ratio, if you change the amount (volume) you are also changing the mass at the same rate.
1/4 = 2/8 = 4/16 = 8/32 they're all the same number because they're all going up by the same on the top AND the same on the bottom.
The boxes that most likely came from the same substance (material) are boxes ____ & _____. (there are two acceptable answers, I only need one)
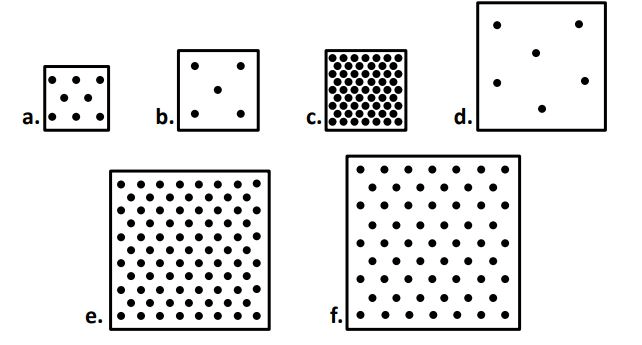
A & F
or
E & F
the least dense object _____
the most dense liquid _____
the soda cap has a similar density to ______
the cherry tomato is more dense than ____ but less dense than _____
the popcorn kernel is _______ than corn syrup but _____ then 100% maple syrup

ping pong ball
honey
rubbing alcohol
water, milk
less dense, more dense
Compare the densities of the purple ball vs the green ball if the liquid they are in has a density of 3 g/mL.

The purple ball is less dense than the green ball because it is floating slightly higher.
Milo finds a cube in his bin of toys and decides to find its density. When he measures one of its sides it comes out to 4 cm and when he places the cube on a scale it measures in at 64 g. Calculate the density of the cube.
V = L x W x H
V = 4 x 4 x 4 (a cube has all the same side lengths. If one side is 4 cm, they're ALL 4 cm)
V = 64 cm3
D = M/V
D = 64/64
D = 1 g/cm3
Sora finds a rock and wants to identify it as Pumice or Lava rock. The two rocks look identical and have similar physical properties so she decides to use their densities to determine the identity. Pumice has a density of 1 g/cm3 whereas Lava Rock has a density of 2 g/cm3. The mystery rock has a mass of 400 grams and since it is an irregular solid, Sora uses the water displacement method to find the volume. Using the photo below, calculate the density and identify whether the rock is Pumice or Lava rock.

V = ending water level - starting water level
V = 800 - 600
V = 200
D = M/V
D = 400/200
D= 2 g/cm3
Mystery rock is Lava rock
Fred measures the volume of a solid object using the water displacement method so his units would be _____. He then calculates the density of that object so the units would be _____.
mL
g/cm3
Explain, in detail, the relationship between temperature and density. You may use the examples discussed in class such as lava lamps, hot air balloons, or the temperature differences between the basement and attic of your house.
Hot substances are less dense and rise while cold substances are more dense and sink.
House: Cold, more dense air sinks to the basement and hot, less dense air rises to the attic.
Lava Lamps: The goo inside the lamp near the light bulb heats up, becomes less dense and rises. When it gets to the top of the lamp away from the heat of the light bulb it cools, becomes more dense, and sinks.
Hot Air Balloon: The fire from the balloon heats up the air inside the balloon making it less dense than the air around the balloon which makes the air balloon rise up. When they're ready to descend, they stop heating up the air inside the balloon.