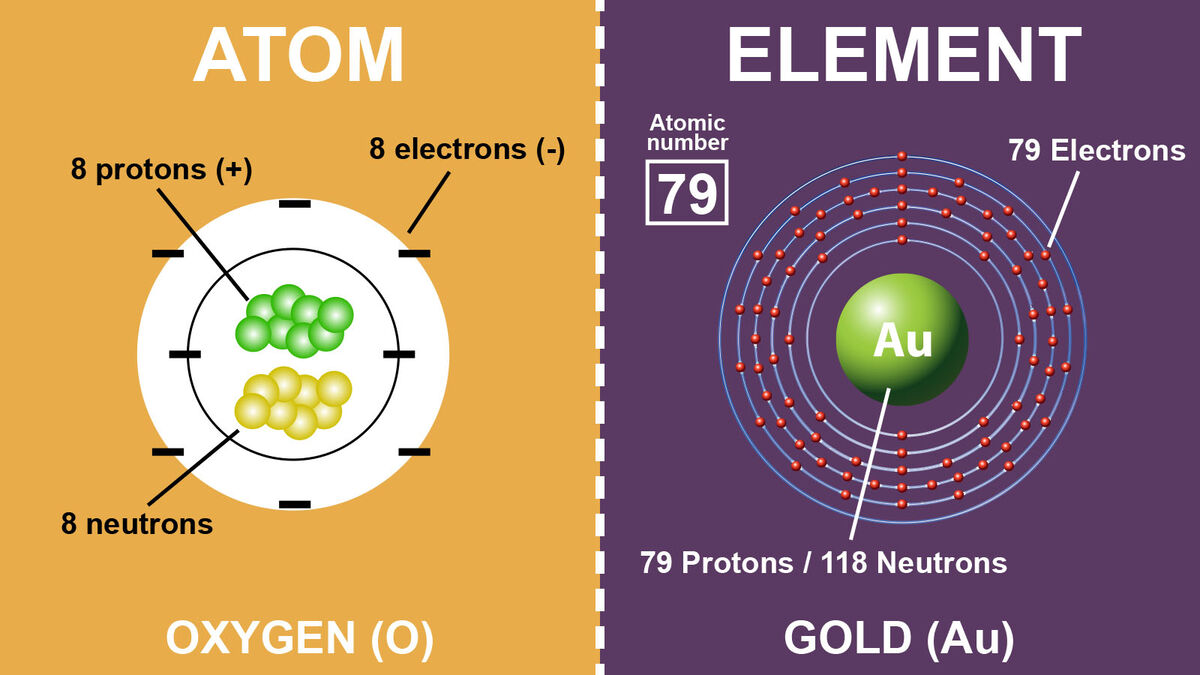
The atomic element 
MI
What is a molecule, and how would you recognize one?
a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance.
Whats in a electron
elementary particles
science is best
yes
oxogen
o.
 lantheume
lantheume
LU
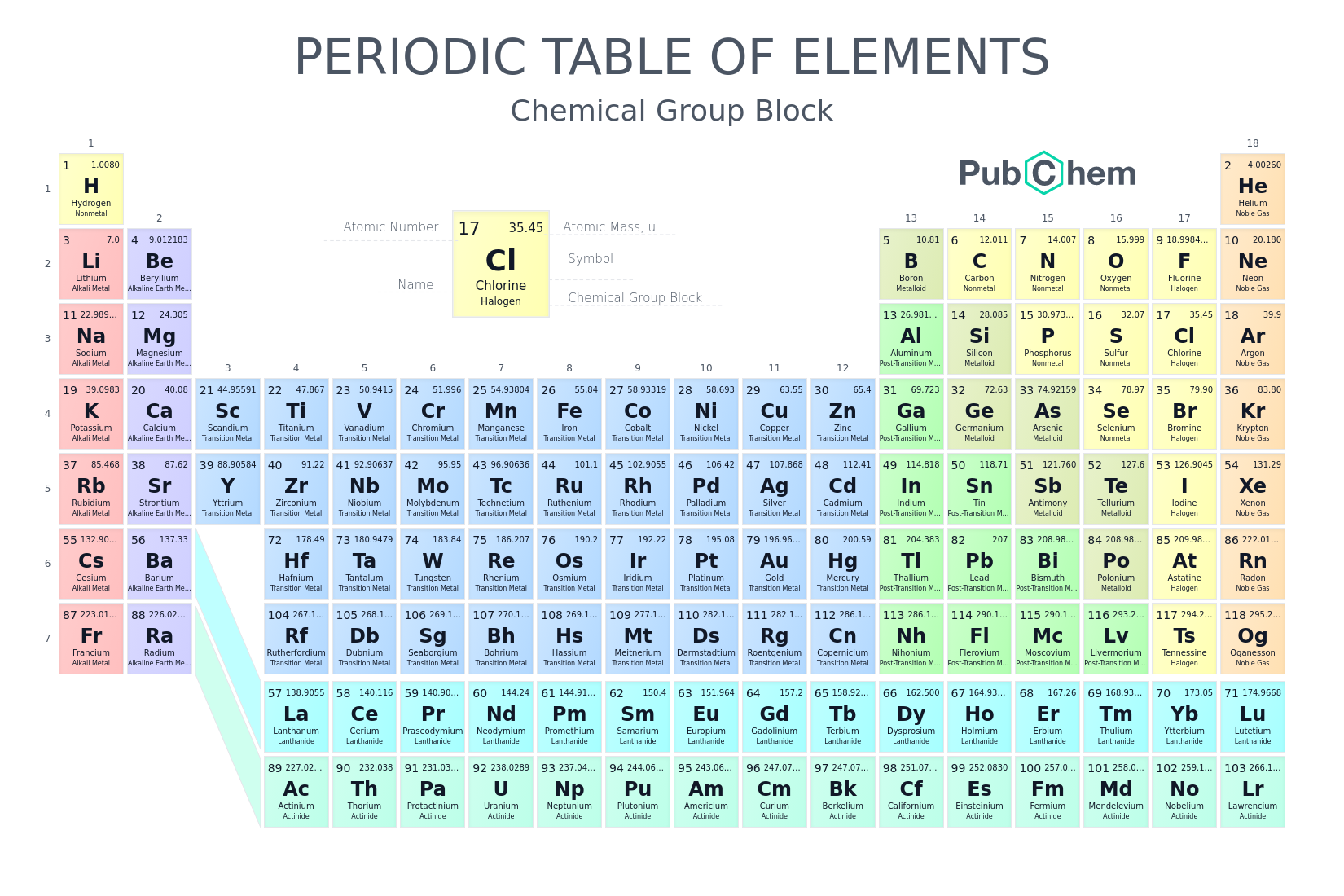
 11 atomic number
11 atomic number
11 protons 11 electrons
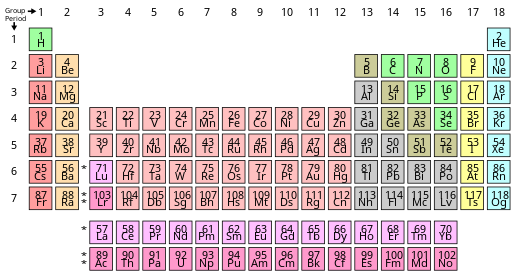
How big are the different parts of an atom? (which pieces are bigger than others?)
Atoms become larger as we go down a column of the periodic table, and they becomes smaller as we go across a row of the table
Whats in a molecule?
two or more atoms connected by chemical bonds
mr knorr is best
yes
helium
h
 Caceum
Caceum
CA
Atomic number is ten what's the neutrons.
subtract atm by mass gets nutons
how many atoms are in liver.
hydrogen, oxygen, nitrogen, sulfur, and phosphorus
Whats the most Important thing about an atom.
the smallest unit into which matter can be divided without the release of electrically charged particles
 iron
iron
fe
 lodine
lodine
L
Scandeum
SC
the mass
at the bottom that usually a digit
whats in a atom?
protons, neutrons, and electrons
Whats the most important part of a molecule
DNA
boron
b
 yttriume
yttriume
Y
MIgraemith
MG
How does static electricity work?
Static electricity is the result of an imbalance between negative and positive charges in an object
whats in a proton?
two up quarks and one down quark,
What dose h2o stand for.
water
nitrogen
N
 vandueim
vandueim
v
Silver
AG