What is the half-life of carbon-14?
5.7 x 103 years
5700 years
Which Radioactive Isotope would be best to use in dating a rock believed to be between 2,000,000,000 (2.0x109) and 3,000,000,000 (3.0x109) years old?
Potassium-40
40K
If an original sample of Carbon-14 had a mass of 10 grams, what amount of Carbon-14 would be left after 11,400 years?
0 Half-Life (Time Zero) - 10g
1 Half-Life (5700 years passed) - 10g/2 = 5g
2 Half-Life (11,400 years passed) - 5g/2 = 2.5g
2.5 grams
Radioactive Decay of Carbon-14 begins when an organism dies.
True
What is the parent material of 87Sr?
rubidium-87
87Rb
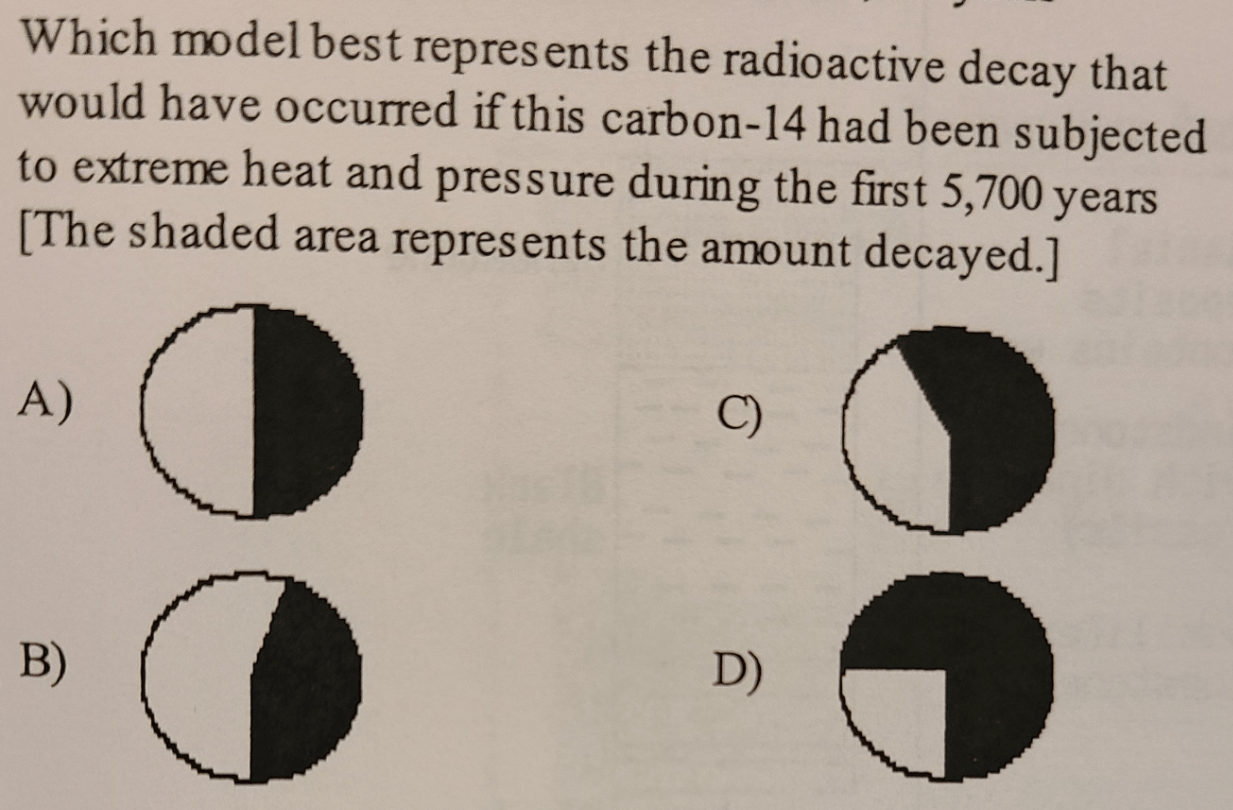
A - heat and pressure do not affect the rate of radioactive decay!
A rock sample was found to have 15g of Potassium (40K) and 45g of Calcium (40Ca). According to the ESRT, how old is the rock sample?
Original Amt of Potassium = 15g +45g = 60g
0 Half-Life (Time Zero) - 60g of Potassium-40
1 Half-Life (1.3x109 years) - 30g of Potassium-40
2 Half-Life (2.6x109 years) - 15g of Potassium-40
2.6x109
2.6x109 years
Absolute and Relative Dating both calculate the age of rocks in years.
False
Absolute Dating provides ages in years, and Relative Dating provides a sequence of geologic events over time.
What is the daughter product of uranium-238?
206Pb
lead-206
A sample of rock contained 100 grams of Potassium-40 (40K) when it was formed. Today the rock contains 50 grams of Potassium-40 (40K). According to the ESRT, what is the age of the rock?
A) 1.3X109 years
Radioactive Carbon-14 (C14) dating has determined that a fossil is 17.1x103 years old. What is the total amount of the original C14 still present in the fossil?
A) 12.5% C) 50%
B) 25% D) 75%
A) 12.5%
0 Half Life (Time Zero) - 100%
1 Half Life (5.7x103) - 50%
2 Half Life (11.4x103) - 25%
3 Half Life (17.1x103) - 12.5%
Carbon-14 Dating is the most appropriate way to determine the age of dinosaur fossils from the Early Cretaceous Period.
False
Carbon-14 Dating can only date relatively young remains...because its half-life is short.
SHORT Half Life - YOUNG material
LONG Half Life - OLD material
Which radioactive isotope has the shortest half-life, and what does it decay into?
carbon-14 --> nitrogen-14
Stretch Your Thinking...
If Absolute Dating is the most accurate method of dating rocks, fossils and organic remains, then why do scientists use other methods of dating, too? (Relative Dating & Correlation)?
Some Reasons:
1. Some rocks are made up of other rocks (Sedimentary Rocks)...More than one Time Zero
2. Some rocks that have been reheated (Metamorphic Rocks) since they were orginally formed...Time Zero may have been reset
3. Cross Verification (Checking your work)
4. Funding
How many half lives will have passed when Carbon-14 decays from 100% to 0% within a rock sample?
Radioactive isotopes will never completely decay. That is why they have the potential to be dangerous! They are never actually gone. Check this out.
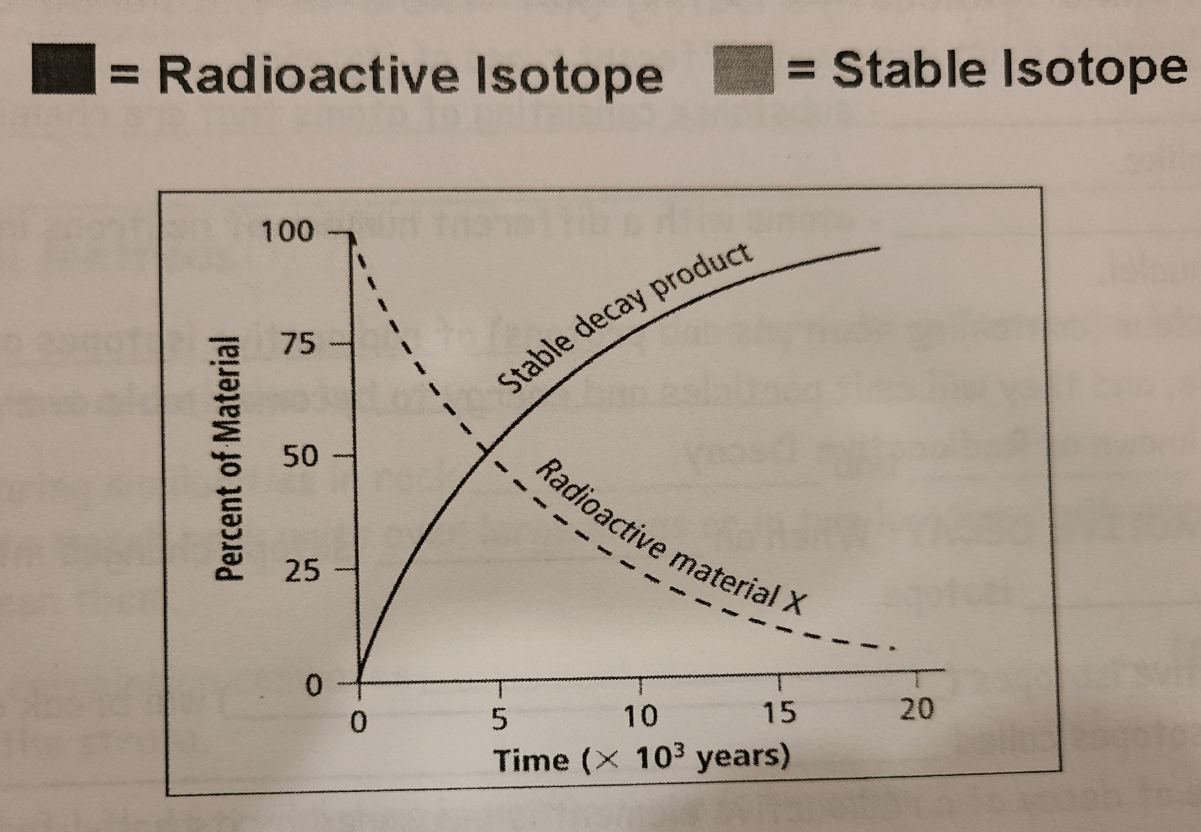
Heat and Pressure CANNOT affect a radioactive isotope's rate of decay.
True
Half lives are CONSTANT
...the RATE of decay NEVER CHANGES